CBSE Class 11-science - Solubility Product Videos
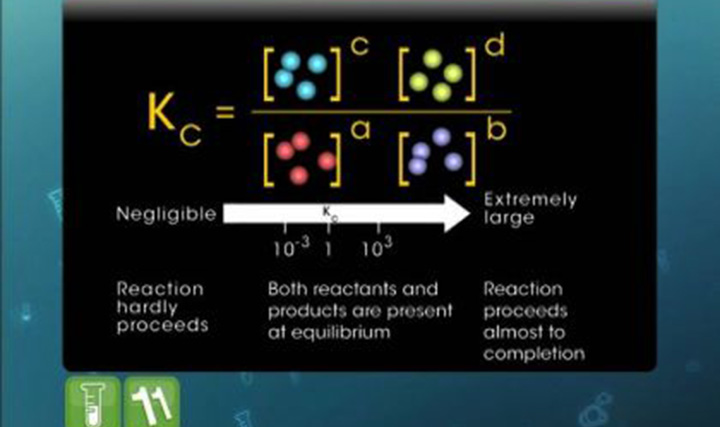
Equilibrium
This video explains solubility, factors influencing solubility, classification of solutes and solubility product.
More videos from this chapter
View All-
Chemistry
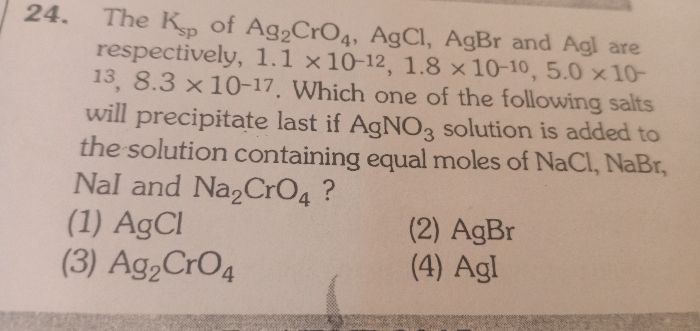
-
Chemistry

-
pls explain

- Q) The solubility of BaSO4 in water is 2.3*10^4gm/100ml. Calculate the percentage loss in weight when 0.2gm of BaSO4 is washed with i) 1L of water ii)1L of 0.01N H2SO4.
-
Which is the correct increasing order of the solubility of silver chloride in 0.1 M solution of the above compounds.
(a) Sodium chloride
(b)sodium nitrate
(c)silver chromate
Options
(1)a<b<c
(2)b<c<a
(3)c<a<b
(4)a<c<b

- what is the value of concentration of [h3o] and [oh] in neutral aqueous solution a 298k?ste their ph and poh value
- what is the minimum pH of a solution 0.1M in Mg^2 from which Mg(OH)2 will not precipiate Ksp = 1.2 * 10%-11M^-3
- What do you mean by solubility product?
- How is solubility different from solubility Product?
- How solubility and solubility products are are related?