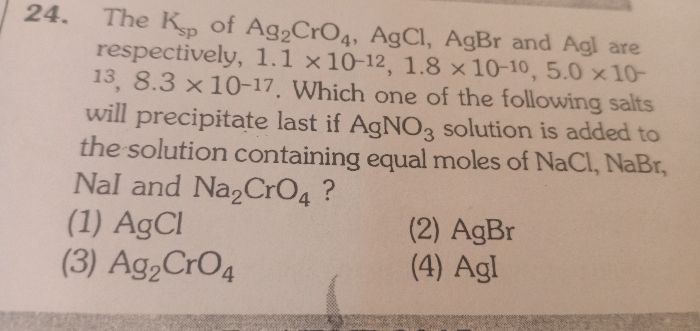CBSE Class 11-science Answered
How solubility and solubility products are are related?
Asked by Topperlearning User | 16 Jun, 2016, 17:24: PM
For a general reaction
AxBy 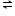 x A y+ + y Bx-
x A y+ + y Bx-
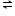 x A y+ + y Bx-
x A y+ + y Bx- Solubility xS yS
Ksp = [x S ]x [y S¯]y = x x y y[S ] x+y
For AB type salt Ksp = S2 or S = (Ksp)1/2,
For AB2/A2B type salt, Ksp = 4S3
or, S = (Ksp/4)1/3 , Where S represents Molar solubility of the salt.
Answered by | 16 Jun, 2016, 19:24: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by achamerahul2 | 21 Apr, 2020, 14:33: PM
CBSE 11-science - Chemistry
Asked by achamerahul2 | 21 Apr, 2020, 14:32: PM
CBSE 11-science - Chemistry
Asked by mufeedatvp2000 | 18 Apr, 2020, 14:21: PM
CBSE 11-science - Chemistry
Asked by Anish | 23 Aug, 2019, 01:48: AM
CBSE 11-science - Chemistry
Asked by jhajuhi19 | 12 Jun, 2019, 19:23: PM
CBSE 11-science - Chemistry
Asked by Prakash | 28 Jun, 2018, 18:09: PM
CBSE 11-science - Chemistry
Asked by gganga | 10 Apr, 2018, 18:31: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 May, 2015, 15:13: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 30 Apr, 2015, 14:30: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 17:24: PM