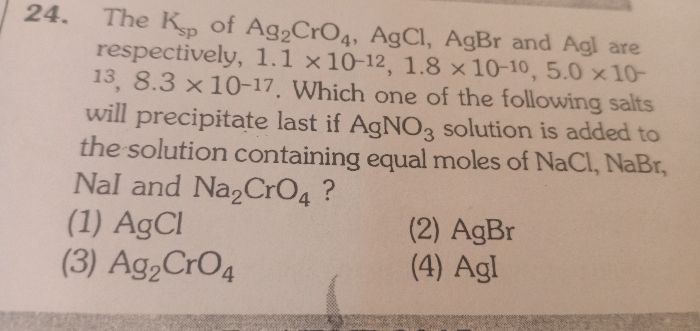CBSE Class 11-science Answered
what is the value of concentration of [h3o] and [oh] in neutral aqueous solution a 298k?ste their ph and poh value
Asked by Prakash | 28 Jun, 2018, 18:09: PM
Water undergoes self-ionisation to a small extent as,
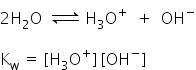
The constant Kw is called as an ionic product of water.
Its value at 298K is 1.008 X 10-14 Mol2 L-2.
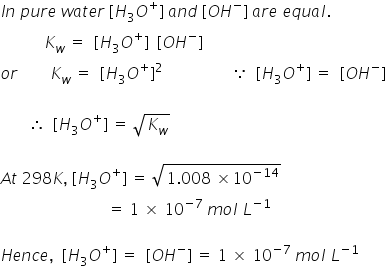
Now, let's find the pH and pOH value,
pH = log[H+]
= log [1 X 10-7]
pH = 7
pH + pOH = 14
7 + pOH = 14
pOH = 7
Answered by Ramandeep | 29 Jun, 2018, 11:25: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by achamerahul2 | 21 Apr, 2020, 14:33: PM
CBSE 11-science - Chemistry
Asked by achamerahul2 | 21 Apr, 2020, 14:32: PM
CBSE 11-science - Chemistry
Asked by mufeedatvp2000 | 18 Apr, 2020, 14:21: PM
CBSE 11-science - Chemistry
Asked by Anish | 23 Aug, 2019, 01:48: AM
CBSE 11-science - Chemistry
Asked by jhajuhi19 | 12 Jun, 2019, 19:23: PM
CBSE 11-science - Chemistry
Asked by Prakash | 28 Jun, 2018, 18:09: PM
CBSE 11-science - Chemistry
Asked by gganga | 10 Apr, 2018, 18:31: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 May, 2015, 15:13: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 30 Apr, 2015, 14:30: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 16 Jun, 2016, 17:24: PM