CBSE Class 11-science - Dynamic Nature of Equilibrium Videos
Equilibrium
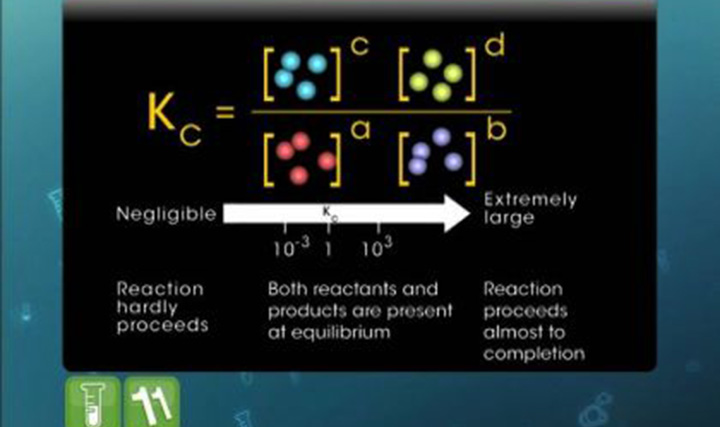
This video explains the dynamic nature of equilibrium.
More videos from this chapter
View All-
1/6.3X10 to the power 14

-
please solve

-
question 54...chemical equilibrium... how to solve.... plz tell

- Explain relationships b/w equilibrium constant K, reaction quotient Q and Gibbs energy G.
- For a reaction, A + B → 2C 2 moles of A and 3 moles of B are allowed to react. If equilibrium constant is 4 at 400° C, then the mole of C at equilibrium is
-
Answer with explanation....Thanks

-
Pls answer the following

- What do you mean by equilibrium state?
- A crystal of common salt of given mass is kept in aqueous solution. After 12 hours, its mass remains the same. Is the crystal in equilibrium with the solution?
- Give three examples of physical equilibria.