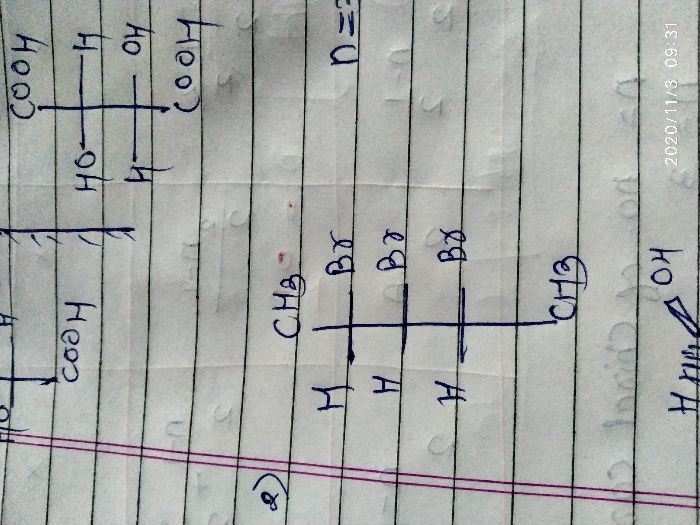CBSE Class 11-science Answered
orthonylclorobenzene and paradiclorobenzene which one have more mealting point and boiling point?
Asked by gargimoitreyee | 20 Mar, 2018, 08:21: PM
The melting point and boiling point of para dichlorobenzene is higher than its ortho and meta isomers.
This is because para chlorobenzene has symmetrical structure and therefore molecules are closely packed in crystal lattice.
These molecules have strong intermolecular attraction.
Due to this, more energy is required to break the bonds.
Answered by Varsha | 21 Mar, 2018, 12:54: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by ap4450962 | 12 Mar, 2024, 07:35: PM
CBSE 11-science - Chemistry
Asked by shahintkjnv2016 | 13 Jun, 2022, 07:17: PM
CBSE 11-science - Chemistry
Asked by rohus442 | 03 Nov, 2020, 09:31: AM
CBSE 11-science - Chemistry
Asked by ashok.amireddi | 01 May, 2020, 10:33: PM
CBSE 11-science - Chemistry
Asked by mufeedatvp2000 | 13 Apr, 2020, 08:36: PM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 12 Dec, 2019, 12:00: AM
CBSE 11-science - Chemistry
Asked by ajaysankhala051 | 02 Sep, 2019, 02:26: PM
CBSE 11-science - Chemistry
Asked by musira29rahman | 30 Aug, 2019, 05:09: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 27 Aug, 2019, 06:33: PM
CBSE 11-science - Chemistry
Asked by ajaysankhala051 | 26 Aug, 2019, 09:39: PM