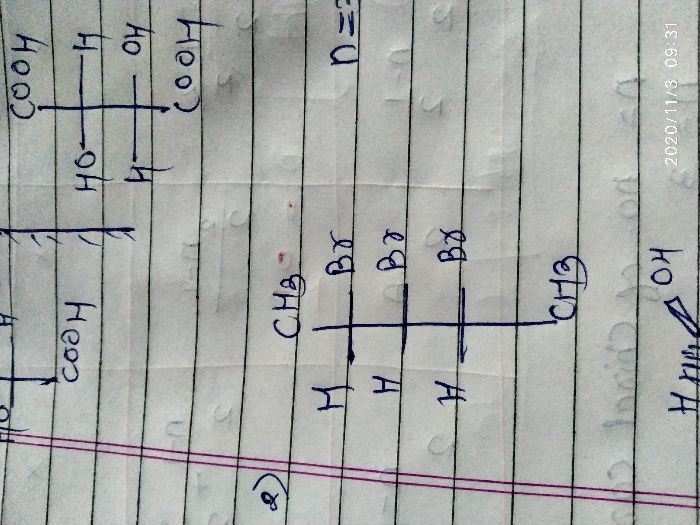CBSE Class 11-science Answered
both of them contain hydrogen bond in their enol form , then why 2 has more enol content than 1

Asked by musira29rahman | 30 Aug, 2019, 05:09: PM
In (1), Number of alpha hydrogen=5
In (2), Number of alpha hydrogen=8
When alpha hydrogen are present in more quantity than more number of enol forms will be formed. So second structure has more enol content than first structure.
Answered by Ravi | 30 Aug, 2019, 06:17: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by ap4450962 | 12 Mar, 2024, 07:35: PM
CBSE 11-science - Chemistry
Asked by shahintkjnv2016 | 13 Jun, 2022, 07:17: PM
CBSE 11-science - Chemistry
Asked by rohus442 | 03 Nov, 2020, 09:31: AM
CBSE 11-science - Chemistry
Asked by ashok.amireddi | 01 May, 2020, 10:33: PM
CBSE 11-science - Chemistry
Asked by mufeedatvp2000 | 13 Apr, 2020, 08:36: PM
CBSE 11-science - Chemistry
Asked by prakriti12oct | 12 Dec, 2019, 12:00: AM
CBSE 11-science - Chemistry
Asked by ajaysankhala051 | 02 Sep, 2019, 02:26: PM
CBSE 11-science - Chemistry
Asked by musira29rahman | 30 Aug, 2019, 05:09: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 27 Aug, 2019, 06:33: PM
CBSE 11-science - Chemistry
Asked by ajaysankhala051 | 26 Aug, 2019, 09:39: PM