CBSE Class 11-science Answered
Give the oxidation number of all elements in CH3COOH? In my book the oxidation number of oxygen in this compound is -1? How
Asked by govtsecschoolnayaganv051 | 06 Aug, 2018, 18:11: PM
rULES FOR ASSIGNING OXIDATION NUMBERS
1. A free element has zero oxidation number
2. Oxygen always has oxidation number -2, except peroxide (H2O2) where it is -1 and with fluorine like OF2 it is +2.
3. When hydrogen is combined with a non-metal then its oxidation number is +1, but in case of metals, its oxidation number is -1.
4. The algebraic sum of oxidation numbers of elements in a compound is zero.
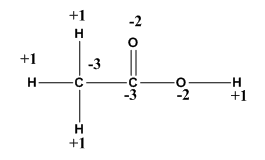
Answered by Ramandeep | 07 Aug, 2018, 11:11: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by Kattaithihaas | 24 Jul, 2022, 18:03: PM
CBSE 11-science - Chemistry
Asked by snehadethe45 | 22 Oct, 2020, 11:31: AM
CBSE 11-science - Chemistry
Asked by defence221175 | 17 Feb, 2020, 18:10: PM
CBSE 11-science - Chemistry
Asked by adalroshan2464 | 12 May, 2019, 15:19: PM
CBSE 11-science - Chemistry
Asked by vishakhachandan026 | 16 Apr, 2019, 11:39: AM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 06 Aug, 2018, 18:11: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Apr, 2015, 14:41: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM






 b) In the reaction
b) In the reaction  , what is oxidised and what is reduced?
, what is oxidised and what is reduced? 
