CBSE Class 11-science Answered
a) Identify the substance oxidised, substance reduced, oxidising agent and reducing agent in the following reaction:
 b) In the reaction
b) In the reaction  , what is oxidised and what is reduced?
, what is oxidised and what is reduced?
 b) In the reaction
b) In the reaction  , what is oxidised and what is reduced?
, what is oxidised and what is reduced?
Asked by adalroshan2464 | 12 May, 2019, 15:19: PM
(a) In this reaction, We need to find oxidation numbers in each compound
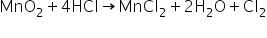
In MnO2,Oxidation number of Mn will be x-4=0
x=+4
In MnCl2,Oxidation number of Mn will be x-2=0
x=+2
So from conversion to MnO2 to MnCl2, Oxidation number is reduced which means MnO2 is reduced to MnCl2 and this process is reduction.
MnO2 is reduced so it will oxidise other compounds.
So MnO2 =Oxidising agent
When HCl is converted to Cl2, it looses hydrogen so when hydrogen is released it means this is Oxidation process and HCl is oxidised which means it will reduce other compounds
So HCl=Reducing agent
(b)
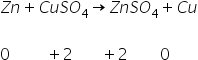
Zn is oxidised in ZnSO4 and CuSO4 is reduced in Cu.
Answered by Ravi | 14 May, 2019, 12:28: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by Kattaithihaas | 24 Jul, 2022, 18:03: PM
CBSE 11-science - Chemistry
Asked by snehadethe45 | 22 Oct, 2020, 11:31: AM
CBSE 11-science - Chemistry
Asked by defence221175 | 17 Feb, 2020, 18:10: PM
CBSE 11-science - Chemistry
Asked by adalroshan2464 | 12 May, 2019, 15:19: PM
CBSE 11-science - Chemistry
Asked by vishakhachandan026 | 16 Apr, 2019, 11:39: AM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 06 Aug, 2018, 18:11: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Apr, 2015, 14:41: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM







