CBSE Class 12-science Answered
Distinguish between polar and non-polar molecules. Give two examples of each.
Asked by Topperlearning User | 23 Apr, 2015, 11:28: AM
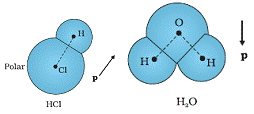
Molecules in which the centres of positive and negative charges are separated even when there is no electric field are called polar molecules. Such molecules have a permanent dipole molecule.
Example: HCl, H2O
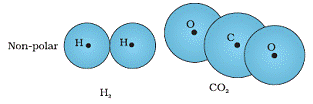
Molecules in which the centres of positive and negative charges coincide in the absence of electric field are called non-polar molecules. Such molecules do not have permanent dipole movement.
Example: H2, CO2
Answered by | 23 Apr, 2015, 13:28: PM
Concept Videos
CBSE 12-science - Physics
Asked by carnivalgirl8421 | 29 Jun, 2022, 11:18: AM
CBSE 12-science - Physics
Asked by priyr7687 | 28 Jun, 2022, 18:19: PM
CBSE 12-science - Physics
Asked by Shrivatsa | 17 Sep, 2018, 15:07: PM
CBSE 12-science - Physics
Asked by abrahamkhan58109 | 16 Aug, 2018, 10:04: AM
CBSE 12-science - Physics
Asked by krishnamittal159 | 18 Jul, 2018, 19:40: PM
CBSE 12-science - Physics
Asked by Topperlearning User | 23 Apr, 2015, 11:25: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 23 Apr, 2015, 11:25: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 23 Apr, 2015, 11:28: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 23 Apr, 2015, 11:29: AM
CBSE 12-science - Physics
Asked by Topperlearning User | 23 Apr, 2015, 11:30: AM




