CBSE Class 11-science Answered
Calculate the 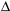 H of the following reaction, which describes the production of coal gas from carbon, given the steps below.
H2O (g)
+
C (s)
H of the following reaction, which describes the production of coal gas from carbon, given the steps below.
H2O (g)
+
C (s)
 CO (g)
+
H2 (g)
Given Steps
H2 (g)
+
1/2 O2 (g)
CO (g)
+
H2 (g)
Given Steps
H2 (g)
+
1/2 O2 (g)
 H2O (g)
H2O (g)
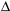 H
=
- 242.0 kJ
2CO (g)
H
=
- 242.0 kJ
2CO (g)
 2 C (s)
+
O2 (g)
2 C (s)
+
O2 (g)
 H
=
+ 221.0 kJ
H
=
+ 221.0 kJ
Asked by Topperlearning User | 15 Jun, 2016, 05:35: PM
1) The overall equation is written above.
Note that the H2O (g) is a reactant and in step #1 it is a product. Thus step one needs to be reversed and the sign of the heat changed.
Note that 1 mole of CO (g) is a product in the overall equation, but 2 moles of CO (g) is a reactant in step 2 . Thus step two needs to be reversed, and divided by two.
Note that 1 mole of CO (g) is a product in the overall equation, but 2 moles of CO (g) is a reactant in step 2 . Thus step two needs to be reversed, and divided by two.
2) Manipulated equations
|
H2O (g)
|
|
H2 (g)
|
+
|
1/2 O2 (g)
|
|
=
|
+ 242.0 kJ
|
|
C (s)
|
+
|
1/2 O2 (g)
|
|
CO (g)
|
|
=
|
- 110.5 kJ
|
3) Add equations and heats
|
H2O (g)
|
+
|
C (s)
|
+
|
1/2 O2 (g)
|
|
1/2 O2 (g)
|
+
|
H2 (g)
|
+
|
CO (g)
|
|
Substances in bold above are common and therefore are canceled
Net Equation:
|
H2O (g)
|
+
|
C (s)
|
|
H2 (g)
|
+
|
CO (g)
|
|
Answered by | 15 Jun, 2016, 07:35: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by paulnaveed202 | 17 Dec, 2020, 12:36: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 13 Aug, 2014, 04:24: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 01:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 09:38: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:36: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 09:58: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:35: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 10:15: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 14 Aug, 2014, 10:41: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 15 Jun, 2016, 05:35: PM



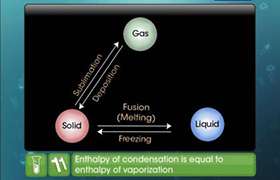
 from: Enthalpy changes of formation and bond enthalpies.
from: Enthalpy changes of formation and bond enthalpies.