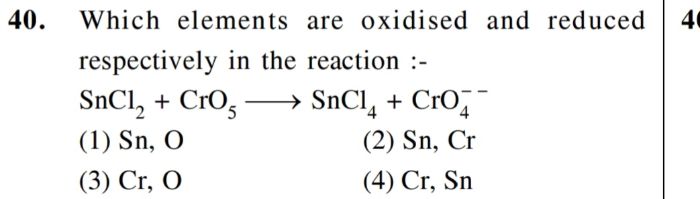CBSE Class 12-science Answered
A green oxide of chromium A on fusion with KOH and KNO3, gives a yellow compound B. The aqueous solution of B on acidification with diluted H2SO4 gives an Orange coloured compound C. Identify A,B,C and write down balanced chemical equations involved.
Asked by mridulabarua05 | 04 Mar, 2019, 11:35: AM
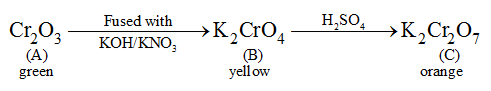
The chemical equations are as follows:
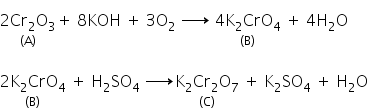
Answered by Ramandeep | 04 Mar, 2019, 12:54: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by anubhutiupadhaya | 27 Feb, 2024, 16:28: PM
CBSE 12-science - Chemistry
Asked by basib61203 | 08 Feb, 2024, 18:03: PM
CBSE 12-science - Chemistry
Asked by ABHILASHA | 04 Mar, 2021, 02:26: AM
CBSE 12-science - Chemistry
Asked by ghoshmahadev037 | 20 Sep, 2020, 11:45: AM
CBSE 12-science - Chemistry
Asked by mufeedatvp2000 | 20 Apr, 2020, 14:53: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 25 Sep, 2019, 22:22: PM
CBSE 12-science - Chemistry
Asked by patra04011965 | 22 Sep, 2019, 13:42: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 30 Aug, 2019, 08:09: AM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 16:46: PM
CBSE 12-science - Chemistry
Asked by Debdulal | 29 Aug, 2019, 16:44: PM