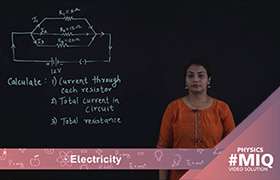
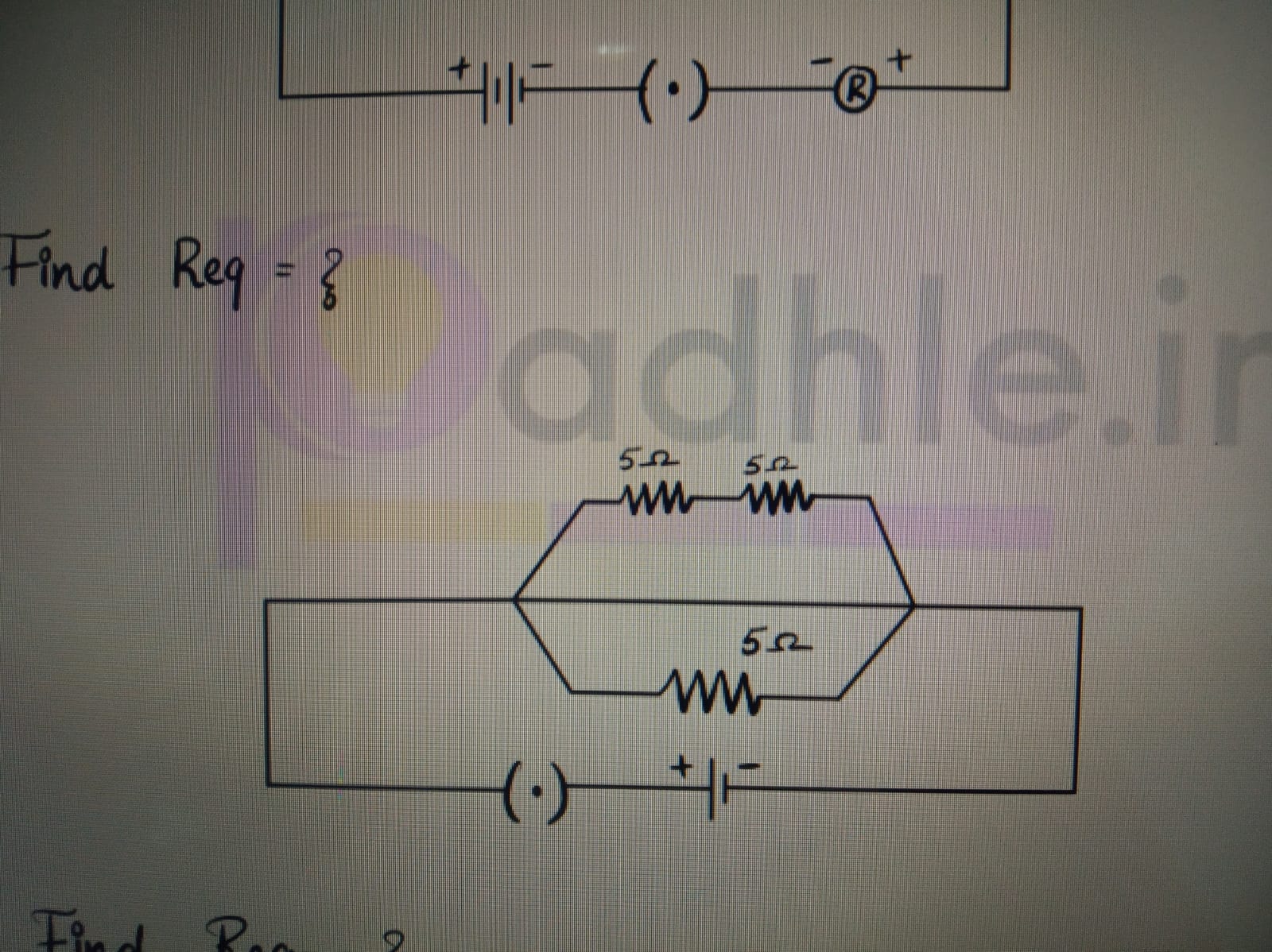
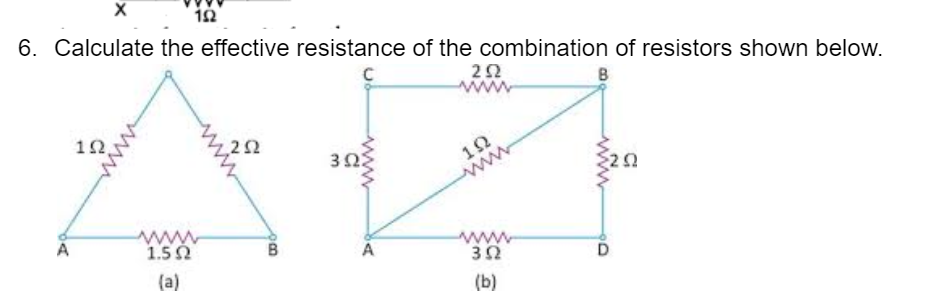
CBSE Class 10 Answered
Neon signs evolved from scientific experiments in which various gases were subjected to high-voltage currents. In 1856, Heinrich Geissler produced a light source by passing a high-voltage alternating current through a low-pressure gas sealed in a glass tube. Subsequent experiments showed that almost all gases would conduct an electric current, and that many would produce light. The problem was that most of the common gases, like carbon dioxide, would react with the current-carrying electrodes within the sealed tube. This quickly reduced the efficiency of the electrodes until the light sputtered and died. In 1898, Sir William Ramsay and Morris William Travers developed a method for the fractional distillation of liquid air. In the process, they discovered the rare gas elements neon, argon, krypton, and xenon. Using these gases in sealed glass tubes, they produced colored light sources ranging from a bright reddish-orange for neon to an intense grayish-blue or violet for argon. Not only did these gases produce colored light, but they were chemically inert and did not react with the electrodes.