CBSE Class 11-science Answered
I do not know how to take the equlibria constant for a heterogeneous equilibrium ...please help me with an example and tell me what to take and what not to.
Asked by reeya tanwar | 17 Nov, 2015, 11:37: PM
For heterogeneous reactions, the concentration terms for pure solids and pure liquids are not included in the expression for equilibrium constant. The concentration of pure solids and liquids remain constant, and these terms are merged into the equilibrium constant or by convention their concentrations are taken as unity, i.e., [solid] = 1, [liquid] = 1.
H2O(g) + C(s) ⇌ H2(g) + CO(g)
So, equilibrium constant can be given as,
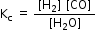 ; [C(s) = 1]
; [C(s) = 1]
Answered by Arvind Diwale | 19 Nov, 2015, 09:58: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by gouravvv641 | 16 Aug, 2022, 09:25: PM
CBSE 11-science - Chemistry
Asked by mangalchandrj79 | 21 May, 2022, 04:38: PM
CBSE 11-science - Chemistry
Asked by veenatripathi | 28 May, 2020, 09:03: AM
CBSE 11-science - Chemistry
Asked by ABHILASHA | 24 Oct, 2019, 06:27: AM
CBSE 11-science - Chemistry
Asked by tanuj2808 | 25 Sep, 2019, 10:18: AM
CBSE 11-science - Chemistry
Asked by jhajuhi19 | 02 Jun, 2019, 11:55: PM
CBSE 11-science - Chemistry
Asked by ntg432000 | 26 Apr, 2019, 04:31: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 24 Apr, 2015, 01:55: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 24 Apr, 2015, 02:07: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 24 Apr, 2015, 02:31: PM









