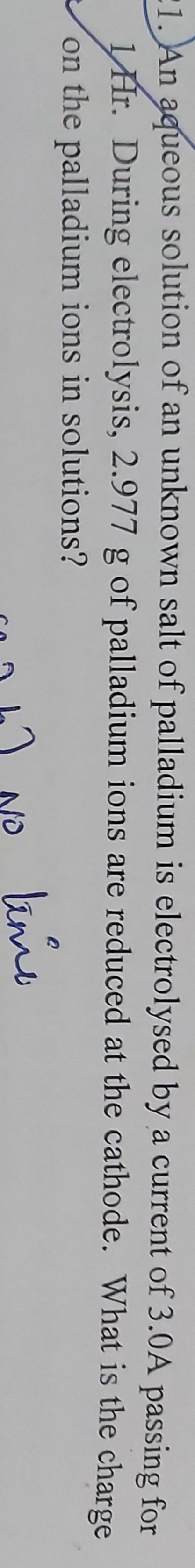CBSE Class 12-science Answered
How much maximum amount of Alumininum metal is deposited at cathode by passing one ampere current for 96500seconds through molten AlCl3?
Asked by sourabh7jain | 07 Jun, 2016, 11:41: AM
Given:
c = 1 ampere
t = 96500 seconds
To find: Weight of Al deposited = ?

Answered by Hanisha Vyas | 09 Jun, 2016, 12:04: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by avaneesh5116 | 07 Aug, 2020, 05:24: PM
CBSE 12-science - Chemistry
Asked by harshpareek696 | 01 Aug, 2020, 04:01: PM
CBSE 12-science - Chemistry
Asked by sulaikhasulu393 | 22 Jun, 2020, 08:52: AM
CBSE 12-science - Chemistry
Asked by vasudesetti123 | 23 May, 2020, 08:13: PM
CBSE 12-science - Chemistry
Asked by sakthisivasakthi1978 | 31 Oct, 2019, 09:53: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:14: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:13: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:12: PM
CBSE 12-science - Chemistry
Asked by lovemaan5500 | 19 Aug, 2019, 11:11: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 19 Jul, 2019, 09:49: PM