CBSE Class 11-science Answered
Explain structures of diborane.
Asked by Topperlearning User | 13 Aug, 2014, 09:10: AM
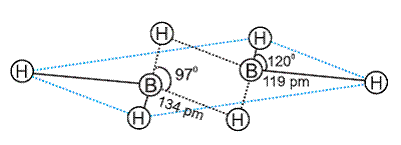
- In the structure of diborane, the four terminal hydrogen atoms and the two boron atoms lie in one plane.
- Above and below this plane, there are two bridging hydrogen atoms.
- The four terminal B-H bonds are regular two centre-two electron bonds while the two bridge (B-H-B) bonds are different and can be described as three centre -two electron bonds or banana bond.
Answered by | 13 Aug, 2014, 11:10: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by ABHILASHA | 08 Sep, 2019, 06:46: PM
CBSE 11-science - Chemistry
Asked by vishakhachandan026 | 12 Jun, 2019, 09:20: AM
CBSE 11-science - Chemistry
Asked by pb_ckt | 28 Apr, 2019, 01:40: PM
CBSE 11-science - Chemistry
Asked by satya785583 | 16 Mar, 2019, 09:18: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 03 Jan, 2019, 01:09: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 03 Jan, 2019, 12:34: PM
CBSE 11-science - Chemistry
Asked by anushkamoudgil123 | 02 Nov, 2018, 05:47: AM
CBSE 11-science - Chemistry
Asked by arunavamitra50 | 04 Jul, 2018, 09:11: PM
CBSE 11-science - Chemistry
Asked by arunavamitra50 | 04 Jul, 2018, 09:10: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 01:10: PM