CBSE Class 11-science Answered
Can dative bond be formed between 2 similar atoms. For example if one oxygen has completed octet by covalent bond with other atom then can this oxygen form dative bond with another oxygen
Asked by govtsecschoolnayaganv051 | 03 Jan, 2019, 01:09: PM
An oxygen atom can form bonds with other two oxygen atoms to form a compound.
This triatomic oxygen molecule i.e. O3 is known as ozone.
Preparation of ozone:
Ozone is generally prepared when silent electric discharge is passed through the pure, cold and dry dioxygen in special apparatus called ozoniser.
During this reaction, only 10% O2 is converted to O3 and product is called ozonised oxygen.

During this reaction, only 10% O2 is converted to O3 and product is called ozonised oxygen.

Formation of ozone is endothermic process hence it is necessary to use silent electric discharge.
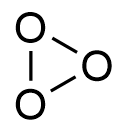
Structure of ozone:
In ozone, the central oxygen atom is sp2 hybridised containing a lone pair of electrons. Hence, ozone has an angular structure with bond angle 1170.
It is actually a resonance hybrid.
Because of resonance, both oxygen-oxygen bonds have partial double bond character.
It is actually a resonance hybrid.
Because of resonance, both oxygen-oxygen bonds have partial double bond character.

Answered by Ramandeep | 03 Jan, 2019, 03:02: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by ABHILASHA | 08 Sep, 2019, 06:46: PM
CBSE 11-science - Chemistry
Asked by vishakhachandan026 | 12 Jun, 2019, 09:20: AM
CBSE 11-science - Chemistry
Asked by pb_ckt | 28 Apr, 2019, 01:40: PM
CBSE 11-science - Chemistry
Asked by satya785583 | 16 Mar, 2019, 09:18: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 03 Jan, 2019, 01:09: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 03 Jan, 2019, 12:34: PM
CBSE 11-science - Chemistry
Asked by anushkamoudgil123 | 02 Nov, 2018, 05:47: AM
CBSE 11-science - Chemistry
Asked by arunavamitra50 | 04 Jul, 2018, 09:11: PM
CBSE 11-science - Chemistry
Asked by arunavamitra50 | 04 Jul, 2018, 09:10: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 31 May, 2016, 01:10: PM