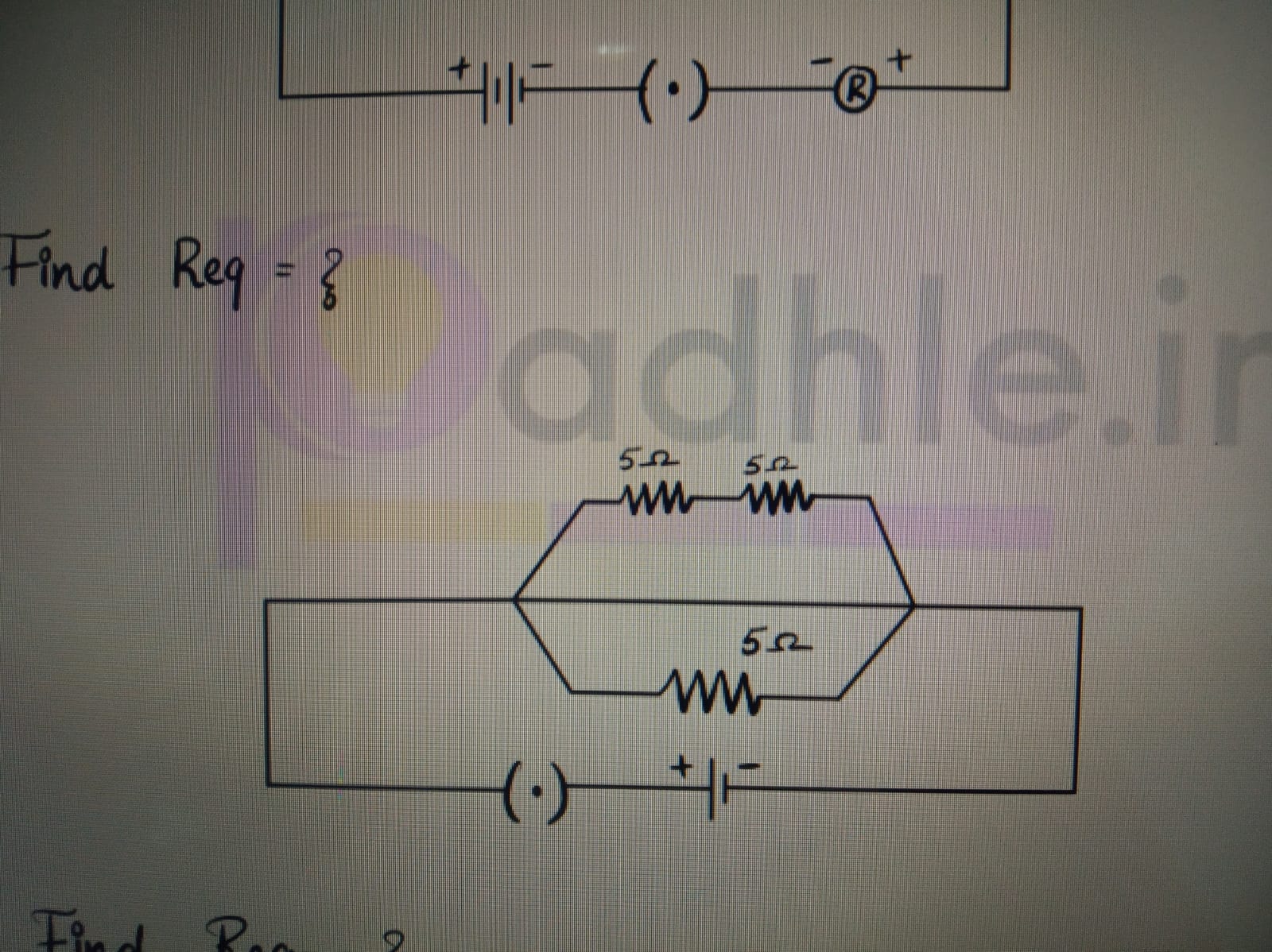
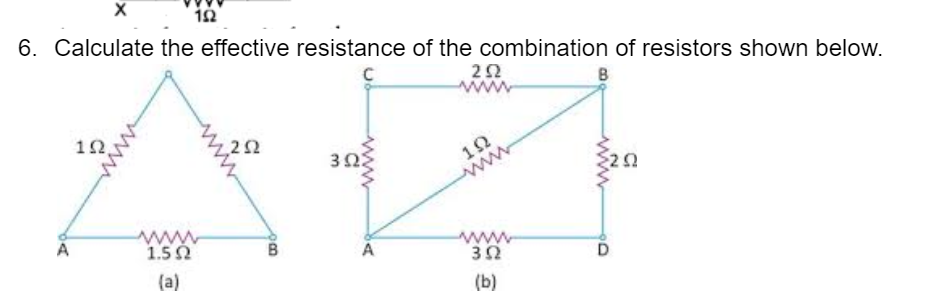
CBSE Class 10 Answered
Do alloys always have higher electrical resistivity , higher melting points and lower electrical conductivity than their pure metals?
Asked by | 15 Sep, 2013, 11:06: AM
Yes
A metal has large number of free electrons and due to this they conduct current and heat.The resistivity if a material or conductor is the hindrance or resistance offered by its structure to the free electrons to flow.
Generally pure metals have low resistivity as they have large number of free electrons and low hindrance due simple atomic arrangement but when two or more metals are combined then the atomic arrangement changes and this changes the number of free electrons as well as increases the hindrance to their flow. Thus alloys have higher resistivity then its constituent metals.
Resistivity of alloys is more than the metal.
Answered by | 16 Sep, 2013, 11:38: AM
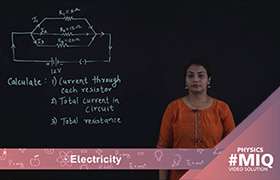
Application Videos
Concept Videos
CBSE 10 - Physics
Asked by khajannirwan | 27 Feb, 2024, 10:20: PM
CBSE 10 - Physics
Asked by saanviyadla | 24 Jan, 2024, 07:06: PM
CBSE 10 - Physics
Asked by kamalaranjanmohantymohanty5 | 06 Jan, 2024, 10:05: AM
CBSE 10 - Physics
Asked by nandhikasugumar | 05 Oct, 2023, 04:01: PM
CBSE 10 - Physics
Asked by daniya062008 | 02 Oct, 2023, 08:25: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:28: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:21: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:13: PM
CBSE 10 - Physics
Asked by prassanna.j | 03 Sep, 2023, 12:11: PM