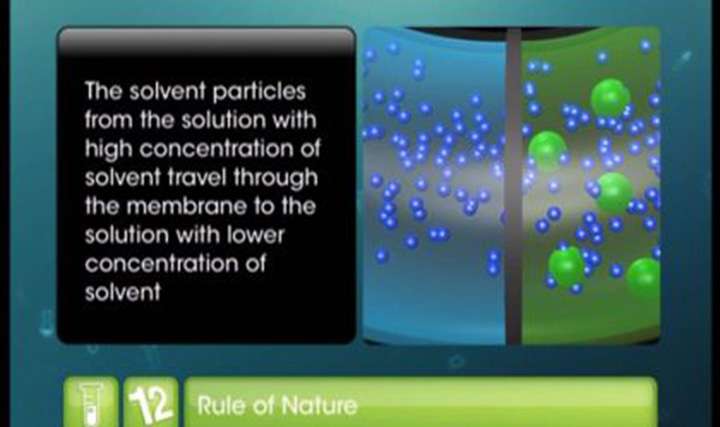
CBSE Class 12-science Chemistry Osmosis and Osmotic Pressure
Go through our well-written topic notes to understand CBSE Class 12 Science Chemistry Solutions – Osmosis and Osmotic Pressure. Find out what is reverse osmosis by watching our chapter videos. In these video lessons, a qualified Chemistry teacher explains the concepts in your syllabus with ample examples. You can rewind these recorded video lessons as many times as required to thoroughly grasp the concept being taught.
For board exam preparation, you should use all the help you can get to study the CBSE Class 12 Science Chemistry Solutions chapter. At TopperLearning, we have compiled the key learning resources to ensure your Chemistry board exam success. These include topic notes, practice tests, mock exam question papers and more.
- Why are equimolar solutions of sodium chloride and glucose not isotonic?
- state of phenomenon of osmosis
-
A 10% solution of urea ( molar mass 60) is found to be isotonic with a 5% solution of an unknown non-electrolyte substance.The molecular mass of unknown substance is
(a) 40%
(b) 50%
(c) 45%
(d) 30%

- The mass of urea that would be dissolved in 180g of water in order to produce the same lowering of vapour pressure as is produced by dissolving 19g of cane sugar (c12 h22 o11) in 100g water is
- vapour pressure of solution of urea is 736.2 mm at 100 degress celsius calculate osmotic pressure of this solution at 15 degress celsius.
- Which is the correct option at same temperature fo r 1 % w/v aqueous solutions of Urea, Glucose and Sugar7 [Molecular Mass : Urea =60 u, Glucose =180 u, Sugar =342 u] (A) n Urea = n Glucose = n Sugar (B) n Urea > n Glucose > n Sugar (C) n Urea > n Sugar > TT Glucose (D) n Sugar > TT Glucose > Jl Urea
- Determine the osmotic pressure of the solution prepared by dissolving 25g of K2SO4 in 2L of water at 25 degree centigrade assuming that it is completely dissociated
-
please solve this

- Osmotic Pressure of 0.4% urea solution is 1.64 atm and that of 3.42% cane sugar is 2.46 atm. When the above two solutions are mixed , the OP of the solution is (a) 0.82 atm (b) 2.46 atm (c) 1.64 atm (d) 4.10 atm
- In barkeley and Hartley method of determining osmotic pressure How the osmotic pressure is balanced by the external pressure so that there is no strain in membrane