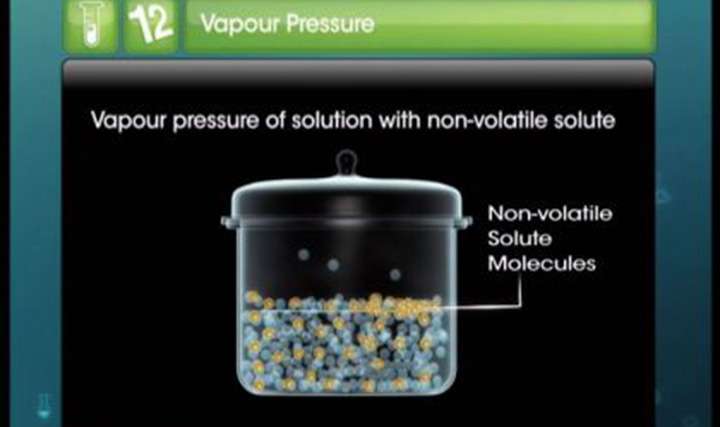
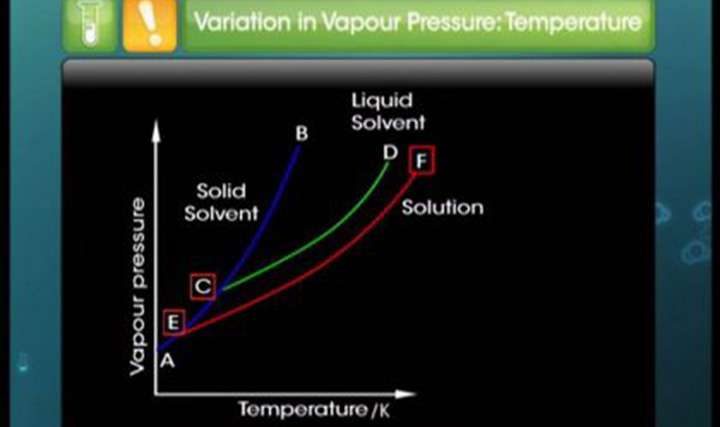
CBSE Class 12-science Chemistry Boiling and Freezing Points of Solutions
Complete your revision of CBSE Class 12 Science Chemistry Solutions – Boiling and Freezing Points of Solutions with TopperLearning’s study materials. For conceptual clarity, check our Chemistry video lessons. Understand what is freezing point depression and what is boiling point elevation through the easy-to-understand explanations by our Chemistry expert in the video lessons.
Revise the most important questions in CBSE Class 12 Science Chemistry through our NCERT solutions and sample paper solutions. To test your conceptual clarity, you should take our practice tests and regularly assess yourself. Such self-evaluation can be used when creating your Chemistry revision plan for the board exam.
- The boiling point of benzene is 353.23k. When 1.80g of a non-volatile solute was dissolved in 90g of benzene , the boiling is raised to 345.11k. Calculate the molar mass of solute (kb=2.53kg mol-1)
-
solution

- Molal elevation constant for benzene is 2.5K/m. A solution of some organic substance in benzene boils at 0.126°C higher than benzene. What is the molality of solution?
- Boiling point of water at 750mm of hg is 99.63°C. how much sucrose is to be added to 500g of water such that it boils at 100°C.
- 2.00g of non-electolyte solute dissolved in 100g of benzene, lowered the freezing point of benzene by 0.30K. find the molar mass of solute.
-
3gm

- What is colligative property
- Explain B.pt.elevation and F.pt.depression?
- To 500 cm3of water ,3×10^-3 of acetic acid is added .if 23 percent is dissociated what will be the depression in freezing pt given k1 is 1.86K kg /mol
- two liquids a and b at 120C and 160C which of these liquids has at higher vapour pressure at 90C