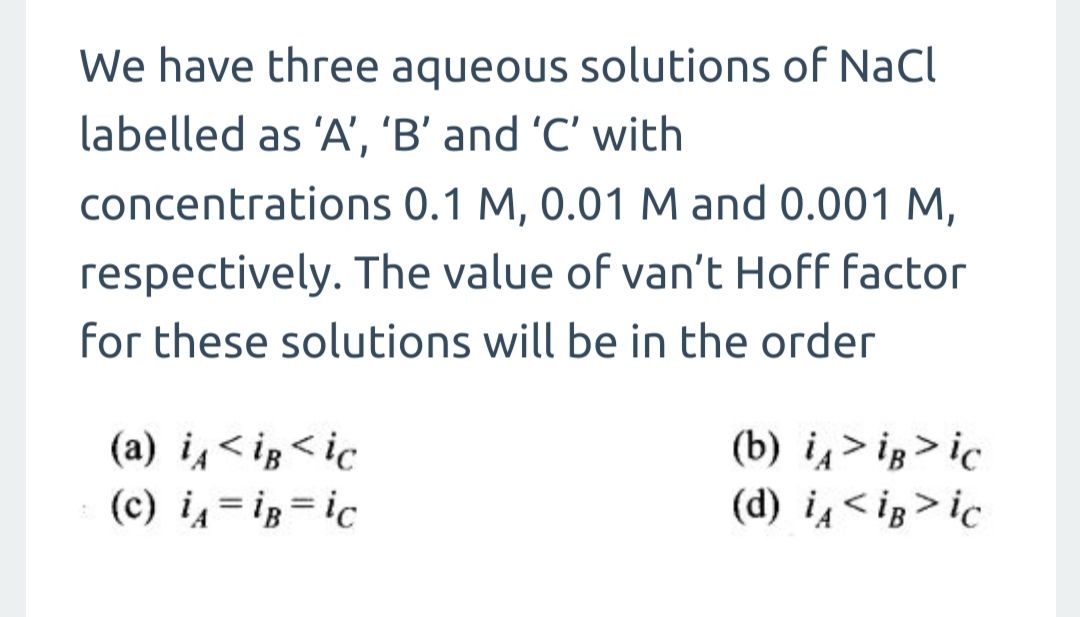
CBSE Class 12-science Chemistry Association and Dissociation
Study CBSE Class 12 Science Chemistry Solutions – Association and Dissociation with TopperLearning’s e-learning resources. Learn about association and dissociation of compounds with our video lessons. Our Chemistry expert will also take you through the concept of abnormal molecular masses in these concept videos. Also, you can access our video lessons and other Chemistry resources online at any time on our study portal.
To practise the topic of association and dissociation in CBSE Class 12 Science Chemistry, check our textbook solutions, practice tests and previous years’ papers. In addition, you can use our sample papers and solutions to easily acquire the answering skills for scoring top marks in your Chemistry board exam.
- The formula of degree of dissociation
- HOW TO FIND VANT OF FACTOR
-
Solution

- Value of Van't Hoff factor if CH3COOH 60% dissociates and 40% dimerized is–(1) 1.2, (2) 1.4, (3) 1.6, (4) 1.8
-
I know that answer is (a) but please explain the reason in detail
- Calculate the freezing point of a solution containing 0.52 g glucose (C6H12O6) dissolved in 80.20g water. For water Kf = 1.86 K Kg mol-1
- 0.90 g of an 1:2 electrolyte was dissolved in 87.90 g of benzene. This raised the boiling point of benzene by 0.250 C. If the molecular mass of the electrolyte is 103.0molar calculate the molal elevation constant for benzene. Given that the solute dissociate with degree of dissociation 0.25.
- 0.216molal solution of Cadmium sulphate is prepared in 1000gram of water. The depression in freezing point was measured to be 0.284K. Calculate the Vant hoff factor. The Cryoscopic constant for water is 1.86K Kg mol-1.
- Two molar solution of potassium Ferro cyanide has degree of dissociation 0.70 at 300K.Find out the osmotic pressure of the solution.
- substance X forms trimer when added to Benzene calculates the freezing point of the 0.25 molal solution. The degree of association of the solute is found to be 0.80. The freezing point of the benzene is 5.5oC and its Cryoscopic constant is 5.12 K m-1.




