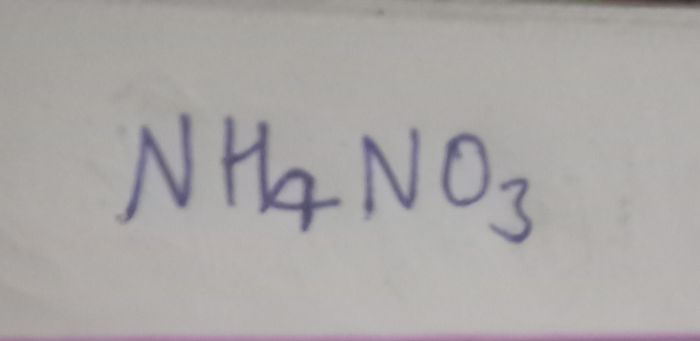CBSE Class 12-science Answered
XeF2 has a liner shape. Why ?
Asked by midhunkrishna | 01 Jul, 2010, 12:09: AM
Xenon has three lone pairs as well as three bonding pairs around it in XeF2. Five electron pairs -----> sp3d hybridization. The lone pairs repel each other more strongly than bonding pairs.
Therefore, it takes the three equatorial positions of the bipyramidal structure of sp3d hybridization, where they can be separated by 120 degrees.
If any one were to occupy the axial position, there would be a 90-degree LP-LP interaction.
Hence, the two bonding pairs occupy the axial positions, and the molecule is linear.
Answered by | 17 Jul, 2010, 10:57: AM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by shwetayaligar205 | 21 Dec, 2022, 07:45: PM
CBSE 12-science - Chemistry
Asked by kushwaharitik9129 | 14 Jul, 2022, 01:06: PM
CBSE 12-science - Chemistry
Asked by gurugubellisaivishal2705 | 09 Jul, 2022, 12:16: PM
CBSE 12-science - Chemistry
Asked by nonuhasan2 | 20 Nov, 2021, 12:05: PM
CBSE 12-science - Chemistry
Asked by skumkum976 | 08 May, 2021, 03:49: PM
CBSE 12-science - Chemistry
Asked by cute44464 | 01 Mar, 2021, 01:17: PM
CBSE 12-science - Chemistry
Asked by manivannanbalakrishnan52 | 09 Dec, 2020, 10:06: PM
CBSE 12-science - Chemistry
Asked by onkaronkar618 | 12 Oct, 2020, 11:38: PM
CBSE 12-science - Chemistry
Asked by contactus.topperlearning | 13 Sep, 2020, 01:21: PM
CBSE 12-science - Chemistry
Asked by nishaohlyan835 | 30 Jun, 2020, 04:18: PM