CBSE Class 12-science Answered
chemical bonding

Asked by manivannanbalakrishnan52 | 09 Dec, 2020, 22:06: PM
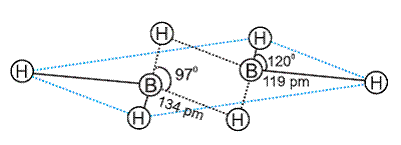
- In the structure of diborane, the four terminal hydrogen atoms and the two boron atoms lie in one plane.
- Above and below this plane, there are two bridging hydrogen atoms.
- The four terminal B-H bonds are regular two centre-two electron bonds while the two bridge (B-H-B) bonds are different and can be described as three centre -two electron bonds or banana bond.
Answered by Ravi | 11 Dec, 2020, 14:24: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by skumkum976 | 08 May, 2021, 15:49: PM
CBSE 12-science - Chemistry
Asked by manivannanbalakrishnan52 | 09 Dec, 2020, 22:06: PM
CBSE 12-science - Chemistry
Asked by onkaronkar618 | 12 Oct, 2020, 23:38: PM
CBSE 12-science - Chemistry
Asked by contactus.topperlearning | 13 Sep, 2020, 13:21: PM
CBSE 12-science - Chemistry
Asked by Daisysnaitz | 24 Apr, 2020, 01:07: AM
CBSE 12-science - Chemistry
Asked by minipkda | 22 May, 2018, 06:42: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 09 Jun, 2014, 16:03: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 11:05: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 09 Jun, 2014, 16:38: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 10 Jun, 2014, 09:24: AM