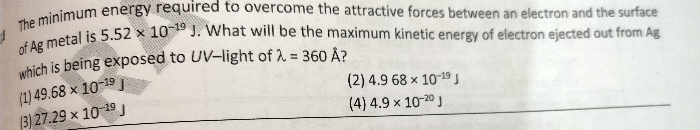CBSE Class 11-science Answered
Why ionisation energy is always positive
Asked by kusumghosh71 | 21 Aug, 2021, 10:59: AM
Ionization energy is the minimum energy required to remove the most loosely bound electron from atom of an element in its ground state. According to IUPAC covention about sign of energy, if energy enters the system, its sign is positive and if energy leaves the system, its sign is negative. In case of ionization energy, energy is always given to the system, hence it is always positive.
Answered by Ravi | 23 Aug, 2021, 11:57: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by sheeltahiliani | 23 May, 2024, 11:58: AM
CBSE 11-science - Chemistry
Asked by saranyachakraborty2007 | 25 Apr, 2024, 05:23: AM
CBSE 11-science - Chemistry
Asked by habibakhatoon112 | 15 Jul, 2022, 21:14: PM
CBSE 11-science - Chemistry
Asked by akankhyapradhan123 | 22 Oct, 2021, 19:29: PM
CBSE 11-science - Chemistry
Asked by kusumghosh71 | 21 Aug, 2021, 10:59: AM
CBSE 11-science - Chemistry
Asked by muramshettyaashrith218 | 16 Sep, 2020, 21:58: PM
CBSE 11-science - Chemistry
Asked by nandanappillai | 21 May, 2020, 13:08: PM
CBSE 11-science - Chemistry
Asked by rajarage18 | 23 Jan, 2020, 20:00: PM
CBSE 11-science - Chemistry
Asked by kuldeepsingh33087 | 27 Jul, 2019, 11:35: AM
CBSE 11-science - Chemistry
Asked by vijaykumartecno348 | 06 Jul, 2019, 21:26: PM