CBSE Class 11-science Answered
why does acidity of hydrides increase as we move from left to right in period?
Asked by imtiyazmulla68 | 23 Mar, 2018, 20:55: PM
On the right-hand side of the row, the acidity increases in the order PH3 < H2S < HCl. This general trend is related to the increasing electronegativity of the element as we move from left to right in any horizontal row. The elctronegativity increases due to which charge separation and ionization of hydrides increases. This increases the acidic strength of hydrides.
Answered by Sivanand Patnaik | 24 Mar, 2018, 11:47: AM
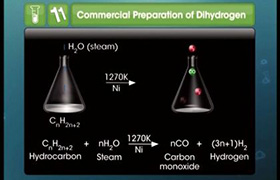
Concept Videos
CBSE 11-science - Chemistry
Asked by mayuresh.sudrik | 25 Sep, 2023, 00:21: AM
CBSE 11-science - Chemistry
Asked by acshanadhana15 | 16 Sep, 2020, 11:17: AM
CBSE 11-science - Chemistry
Asked by ombhattarai693 | 27 Feb, 2020, 20:44: PM
CBSE 11-science - Chemistry
Asked by vatsal012345 | 09 Dec, 2019, 23:09: PM
CBSE 11-science - Chemistry
Asked by Sana3sayeed6394 | 13 Mar, 2019, 13:16: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 27 Sep, 2018, 17:10: PM
CBSE 11-science - Chemistry
Asked by govtsecschoolnayaganv051 | 27 Sep, 2018, 17:08: PM
CBSE 11-science - Chemistry
Asked by mbinukurian1971 | 17 Jun, 2018, 15:16: PM
CBSE 11-science - Chemistry
Asked by imtiyazmulla68 | 23 Mar, 2018, 20:55: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM