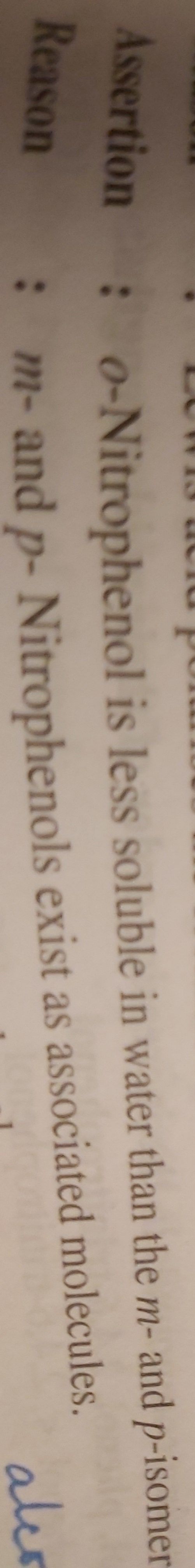CBSE Class 12-science Answered
why do phenols not give the protonation reaction readily ? explain with reaction
Asked by yash.bisht9910 | 17 Jun, 2015, 01:11: PM
The lone pair on oxygen of O-H in phenol is being shared with benzene ring through resonance. Thus, lone pair is not fully present on oxygen and hence phenols do not undergo protonation reactions.
Answered by Prachi Sawant | 17 Jun, 2015, 05:03: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kavitabawane190 | 08 Mar, 2024, 05:24: PM
CBSE 12-science - Chemistry
Asked by rp0055293 | 07 Feb, 2024, 08:28: AM
CBSE 12-science - Chemistry
Asked by vipulverma | 14 Feb, 2022, 04:44: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 23 Jun, 2021, 08:02: PM
CBSE 12-science - Chemistry
Asked by Rg598555 | 30 Oct, 2019, 10:35: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 30 Aug, 2019, 04:13: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Mar, 2014, 03:43: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 03:12: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 03:17: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Mar, 2014, 04:54: PM