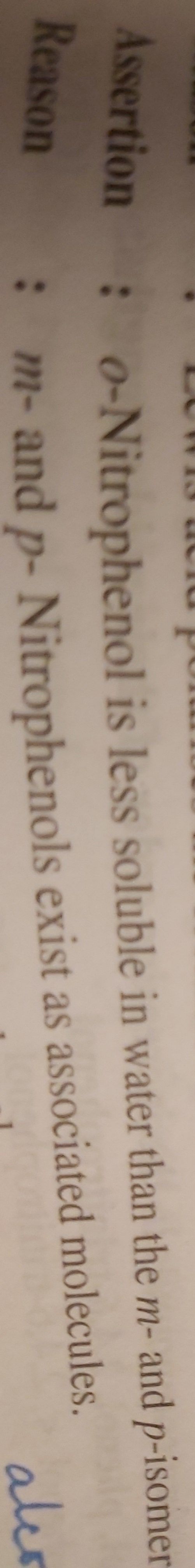CBSE Class 12-science Answered
sir/madam pls help me to answer both the questions plssss......

Asked by kaziryan.05 | 23 Jun, 2021, 20:02: PM
(a)P-nitrophenol> o-nitrophenol > m-nitrophenol.
Nitro group has both M-effect (mesmeric effect) and I-effect (inductive Effect) but M-effect predominates over I-effect. NO2 group at o and p position withdraws electron of o-h bond towards it by stronger M-effect while the NO2 group at m position withdraws electron of o-h bond by weaker I -effect . Thus o and p are nitrophenols are more acidic than m-nitrophenol.
However o-nitrophenol is little less acidic than p-nitrophenol due to intramolecular h-bonding which makes loss of proton little more difficult... So, p-nitrophenol is strongest.
Nitro group has both M-effect (mesmeric effect) and I-effect (inductive Effect) but M-effect predominates over I-effect. NO2 group at o and p position withdraws electron of o-h bond towards it by stronger M-effect while the NO2 group at m position withdraws electron of o-h bond by weaker I -effect . Thus o and p are nitrophenols are more acidic than m-nitrophenol.
However o-nitrophenol is little less acidic than p-nitrophenol due to intramolecular h-bonding which makes loss of proton little more difficult... So, p-nitrophenol is strongest.
(b)m-cresol m > p cresol > o-cresol
m-cresol is acidic and can be explained on the basis of hyper conjugation .
The methyl group leads to increase in electron density on oxygen atom.The ion that is formed as a result of loosing proton is not that stable in case of ortho and para forms .Due to this meta is more acidic .
m-cresol is acidic and can be explained on the basis of hyper conjugation .
The methyl group leads to increase in electron density on oxygen atom.The ion that is formed as a result of loosing proton is not that stable in case of ortho and para forms .Due to this meta is more acidic .
Answered by Ravi | 24 Jun, 2021, 12:34: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kavitabawane190 | 08 Mar, 2024, 17:24: PM
CBSE 12-science - Chemistry
Asked by rp0055293 | 07 Feb, 2024, 08:28: AM
CBSE 12-science - Chemistry
Asked by vipulverma | 14 Feb, 2022, 16:44: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 23 Jun, 2021, 20:02: PM
CBSE 12-science - Chemistry
Asked by Rg598555 | 30 Oct, 2019, 22:35: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 30 Aug, 2019, 16:13: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Mar, 2014, 15:43: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 15:12: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 15:17: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Mar, 2014, 16:54: PM