CBSE Class 12-science Answered
Pl ans sir ...
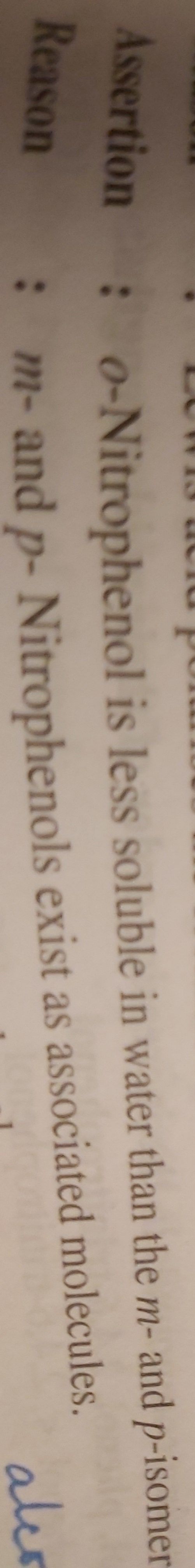
Asked by jain.pradeep | 30 Aug, 2019, 16:13: PM
O-nitrophenol has intramolecular Hydrogen bonding. When it is dissolved in water it is less because it does not form intermolecular hydrogen bond with water.
m-nitrophenol and p-nitrophenol forms intermolecular Hydrogen bond with water so it is more soluble. So Assertion is correct.
Reason- m and p nitro phenol exists as associated molecule due to their intermolecular hydrogen bonding.
So Assertion and Reason both are true but reason is not explanation of Assertion.
Answered by Ravi | 30 Aug, 2019, 19:11: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kavitabawane190 | 08 Mar, 2024, 17:24: PM
CBSE 12-science - Chemistry
Asked by rp0055293 | 07 Feb, 2024, 08:28: AM
CBSE 12-science - Chemistry
Asked by vipulverma | 14 Feb, 2022, 16:44: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 23 Jun, 2021, 20:02: PM
CBSE 12-science - Chemistry
Asked by Rg598555 | 30 Oct, 2019, 22:35: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 30 Aug, 2019, 16:13: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Mar, 2014, 15:43: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 15:12: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 15:17: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Mar, 2014, 16:54: PM





