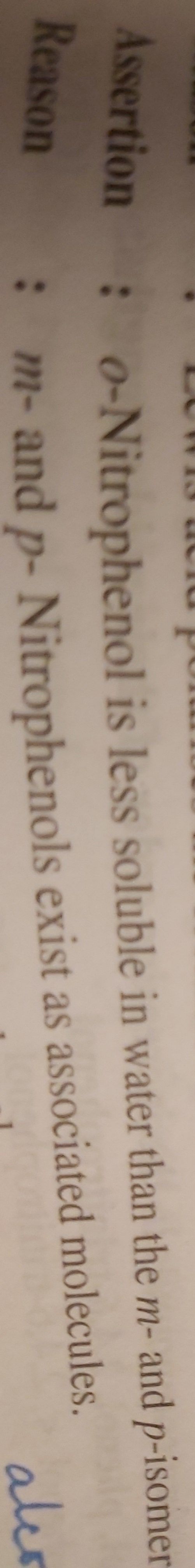CBSE Class 12-science Answered
Which one among alcohols and phenols are more acidic and why?
Asked by Topperlearning User | 07 Jun, 2016, 15:12: PM
Phenoxide is better stabilized than phenol as it carries only a negative charge and phenol has a strong tendency to form more stable phenoxide ion by the release of proton.
But, in case of alcohol both the alcohol molecule and the alkoxide ion are represented by one structure each and there is no resonance.
Also the alkoxide ion due to presence of a formal negative charge has greater energy and is less stable than the alcohol molecule.Thus alcohol have negligible tendency to form less stable alkoxide ion by releasing a proton.
Thus Phenols are stronger acids than alcohol because in case of phenol the phenoxide ion left after the release of a proton is stabilized by resonance.
Answered by | 07 Jun, 2016, 17:12: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by kavitabawane190 | 08 Mar, 2024, 17:24: PM
CBSE 12-science - Chemistry
Asked by rp0055293 | 07 Feb, 2024, 08:28: AM
CBSE 12-science - Chemistry
Asked by vipulverma | 14 Feb, 2022, 16:44: PM
CBSE 12-science - Chemistry
Asked by kaziryan.05 | 23 Jun, 2021, 20:02: PM
CBSE 12-science - Chemistry
Asked by Rg598555 | 30 Oct, 2019, 22:35: PM
CBSE 12-science - Chemistry
Asked by jain.pradeep | 30 Aug, 2019, 16:13: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Mar, 2014, 15:43: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 15:12: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 07 Jun, 2016, 15:17: PM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 27 Mar, 2014, 16:54: PM