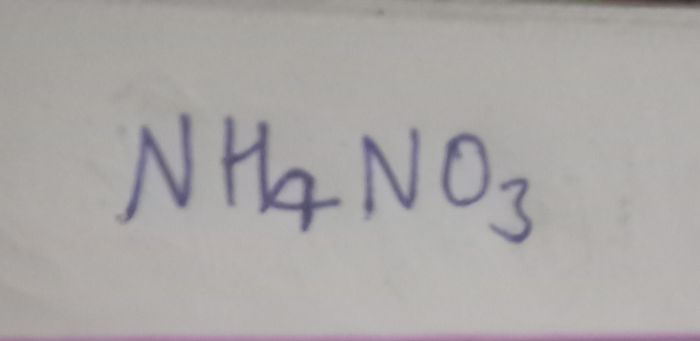CBSE Class 12-science Answered
What is the lone pair of electrons doing in phosphine (PH3) ?? I mean phosphine is stable by having the argon configuration.But what is the significance of the lone pair of electrons in phosphine here?? Pls Explain
Asked by GeoffreyRichards | 10 Nov, 2010, 09:14: AM
Yes, PH3 is stable as they attain their stable electronic configuration but when you decide the shape of the molecule than these lone pairs of electrons contribute towards the shape of the molecule.
Answered by | 10 Nov, 2010, 05:38: PM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by shwetayaligar205 | 21 Dec, 2022, 07:45: PM
CBSE 12-science - Chemistry
Asked by kushwaharitik9129 | 14 Jul, 2022, 01:06: PM
CBSE 12-science - Chemistry
Asked by gurugubellisaivishal2705 | 09 Jul, 2022, 12:16: PM
CBSE 12-science - Chemistry
Asked by nonuhasan2 | 20 Nov, 2021, 12:05: PM
CBSE 12-science - Chemistry
Asked by skumkum976 | 08 May, 2021, 03:49: PM
CBSE 12-science - Chemistry
Asked by cute44464 | 01 Mar, 2021, 01:17: PM
CBSE 12-science - Chemistry
Asked by manivannanbalakrishnan52 | 09 Dec, 2020, 10:06: PM
CBSE 12-science - Chemistry
Asked by onkaronkar618 | 12 Oct, 2020, 11:38: PM
CBSE 12-science - Chemistry
Asked by contactus.topperlearning | 13 Sep, 2020, 01:21: PM
CBSE 12-science - Chemistry
Asked by nishaohlyan835 | 30 Jun, 2020, 04:18: PM