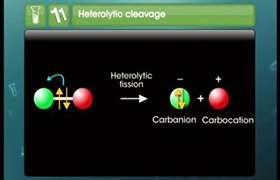
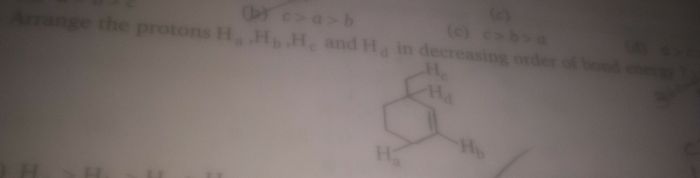CBSE Class 11-science Answered
Rules of drawing resonance structures:
1. Keep atoms where they are at
2. To start with, draw in all hydrogens and lone pairs.
3. π bond electrons or non-bonding pair of electrons readily participate in resonance
4. Therefore, lone pairs on oxygen, halogens, nitrogen, etc and anions can participate in resonance.
5. The total number of electrons does not change.
6. Do not exceed the octet rule in any atom.
Rules of moving electrons in resonance structures:
1. π electrons move towards a positive (+) charge
2. Non-bonding pair (or single electron/radical) moves towards a π bond
3. A non-bonding pair moves towards a positive (+) charge
4. π electrons move towards π bond
5. π electrons move up onto a heteroatom