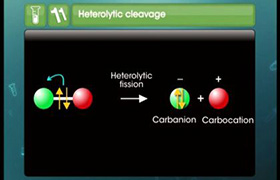
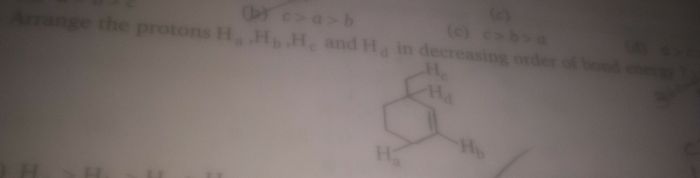CBSE Class 11-science Answered
how to find this
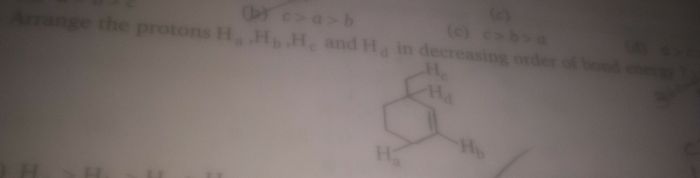
Asked by Subramanian.jayanth | 16 Jun, 2020, 10:39: AM
Dear Students,
In these type of questions, you need to look at bond energies. If breaking of bonds create an unstable compound, so C-Hb has the highest bond energy, because after breaking it results to the formation of a carbocation on double bond, so it makes compound unstable.
If breaking a bond forms any stable carbocation. They will easily break the bond to attain that stable structure. So, in given compounds C-Hd breakage will form most stable carbocation because it involves conjugation and hyperconjugation.
On the basis of this concept, Order would be-
Hb > Hc >Ha >Hd
Answered by Ravi | 16 Jun, 2020, 11:34: AM
Concept Videos
CBSE 11-science - Chemistry
Asked by josephineanto1960 | 28 Mar, 2024, 12:50: PM
CBSE 11-science - Chemistry
Asked by up558486 | 28 Oct, 2021, 12:41: PM
CBSE 11-science - Chemistry
Asked by deepthibai74 | 01 Mar, 2021, 11:13: AM
CBSE 11-science - Chemistry
Asked by bhaleraoshweta237 | 20 Oct, 2020, 21:29: PM
CBSE 11-science - Chemistry
Asked by Subramanian.jayanth | 16 Jun, 2020, 10:39: AM
CBSE 11-science - Chemistry
Asked by kpbhake | 19 Oct, 2018, 18:44: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 18 Jul, 2014, 09:17: AM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM
CBSE 11-science - Chemistry
Asked by Topperlearning User | 04 Jun, 2014, 13:23: PM