CBSE Class 12-science Answered
what are octahedral voids?
Asked by ak4450120 | 08 Apr, 2017, 10:36: AM
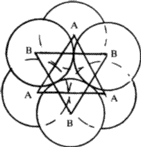
Octahedral void
In the same packing one half of the triangular voids in the first layer are occupied by spheres in the second layer while the other half remains unoccupied. The triangular voids ‘b’ in the first layer is overlapped by the triangular voids in the second layer. The interstitial void, formed by combination of two triangular voids of the first and second layer is called octahedral void because this is enclosed between six spheres centres of which occupy corners of a regular octahedron.
The difference:
In tetrahedral voids---In a close packing, the number of tetrahedral void is double the number of spheres, so there are two tetrahedral voids for each sphere .
In a multi layered close packed structure , there is a tetrahedral hole above and below each atom hence there is twice as many tetrahedral holes as there are in close packed atoms
Radius of the tetrahedral void relative to the radius of the sphere is 0.225.
In octahedral Voids----In close packing, the number of octahedral voids is equal to the number of spheres. Thus, there is only one octahedral void associated with each sphere. Radius of the octahedral void in relation to the radius of the sphere is 0.414.
Answered by Vaibhav Chavan | 08 Apr, 2017, 12:10: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by sardaarjii2012 | 31 Oct, 2021, 09:05: PM
CBSE 12-science - Chemistry
Asked by karennavarpriya | 20 Aug, 2021, 01:53: PM
CBSE 12-science - Chemistry
Asked by apoorvaks26 | 10 May, 2021, 09:24: AM
CBSE 12-science - Chemistry
Asked by someshmule5 | 05 Jan, 2021, 01:25: PM
CBSE 12-science - Chemistry
Asked by deepakatur454 | 16 Oct, 2020, 01:18: PM
CBSE 12-science - Chemistry
Asked by hemanthkumarbk2356 | 02 Aug, 2020, 12:16: PM
CBSE 12-science - Chemistry
Asked by harishshrivastava855 | 21 Jun, 2020, 09:26: PM
CBSE 12-science - Chemistry
Asked by manjurana04933 | 16 Apr, 2020, 11:00: AM
CBSE 12-science - Chemistry
Asked by Saransekar407 | 11 Mar, 2019, 06:47: PM
CBSE 12-science - Chemistry
Asked by yaag8432 | 05 Mar, 2019, 07:28: PM