CBSE Class 12-science Answered
Derive the formula of the ferric oxide
Asked by Saransekar407 | 11 Mar, 2019, 18:47: PM
Question:
Ferric oxide crystallizes in a hexagonal close-packed array of oxide ions with two out of every three octahedral holes occupied by ferric ions. Derive the formula of the ferric oxide.
Answer:
A unit cell of hcp structure = 6
No. of oxide ions per unit cell = 6
No. of octahedral voids = 6 Since, ferric ions occupy only two out of every three octahedral voids,
Therefore, no. of octahedral holes occupied by ferric ions = 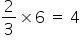
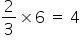
Stoichiometric ratio of Fe : O = 4:6 = 2:3 Hence, formula of ferric oxide = Fe2O3.
Answered by Ramandeep | 11 Mar, 2019, 19:24: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by sardaarjii2012 | 31 Oct, 2021, 21:05: PM
CBSE 12-science - Chemistry
Asked by karennavarpriya | 20 Aug, 2021, 13:53: PM
CBSE 12-science - Chemistry
Asked by apoorvaks26 | 10 May, 2021, 09:24: AM
CBSE 12-science - Chemistry
Asked by someshmule5 | 05 Jan, 2021, 13:25: PM
CBSE 12-science - Chemistry
Asked by deepakatur454 | 16 Oct, 2020, 13:18: PM
CBSE 12-science - Chemistry
Asked by hemanthkumarbk2356 | 02 Aug, 2020, 12:16: PM
CBSE 12-science - Chemistry
Asked by harishshrivastava855 | 21 Jun, 2020, 21:26: PM
CBSE 12-science - Chemistry
Asked by manjurana04933 | 16 Apr, 2020, 11:00: AM
CBSE 12-science - Chemistry
Asked by Saransekar407 | 11 Mar, 2019, 18:47: PM
CBSE 12-science - Chemistry
Asked by yaag8432 | 05 Mar, 2019, 19:28: PM




