CBSE Class 12-science - Crystalline and Amorphous Solids Videos
Crystalline and Amorphous Solids
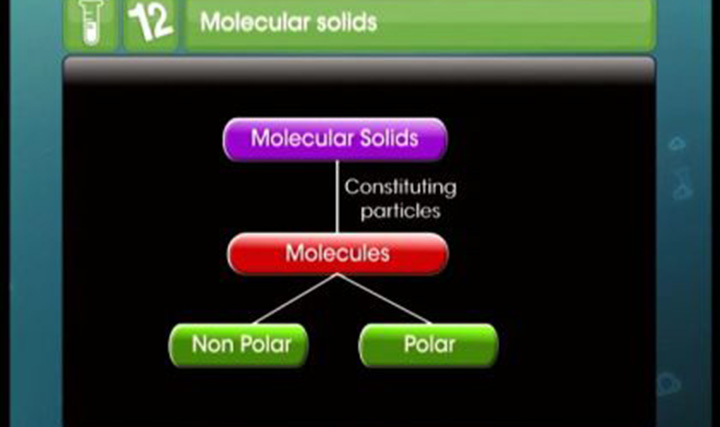
Crystalline and amorphous solids
More videos from this chapter
View All- In diamond C atoms occupy FCC lattice points as well as alternate tetrahedral voids the edge length of unit of unit cell is 356.7pm.Calculate the closest distances between C atom and the fraction of total volume occupies by C atom.
- metal crystallises in BCC structures atomic mass of the metal is 55.8g\mol calculate edge length of unit cell of the metal
- which ionic compound shows both frenkle and schottky defect
- what craystalline solid
- why thionyl chloride method in the best method for prepare alkyl chloride
- what is unit cell
- write distinguish feature of covalent solids?
- unit cell
- Derive the formula of the ferric oxide
- give reason crystaline solid are anisotropic