CBSE Class 12-science Answered
metal crystallises in BCC structures atomic mass of the metal is 55.8g\mol calculate edge length of unit cell of the metal
Asked by karennavarpriya | 20 Aug, 2021, 13:53: PM
Edge length of unit cell of the metal is calculated by-
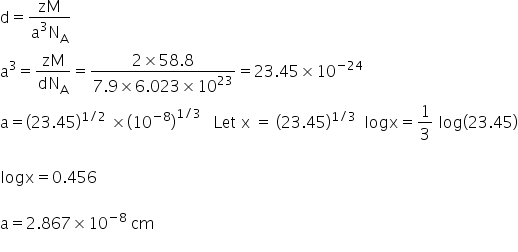
Answered by Ravi | 24 Aug, 2021, 12:34: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by sardaarjii2012 | 31 Oct, 2021, 21:05: PM
CBSE 12-science - Chemistry
Asked by karennavarpriya | 20 Aug, 2021, 13:53: PM
CBSE 12-science - Chemistry
Asked by apoorvaks26 | 10 May, 2021, 09:24: AM
CBSE 12-science - Chemistry
Asked by someshmule5 | 05 Jan, 2021, 13:25: PM
CBSE 12-science - Chemistry
Asked by deepakatur454 | 16 Oct, 2020, 13:18: PM
CBSE 12-science - Chemistry
Asked by hemanthkumarbk2356 | 02 Aug, 2020, 12:16: PM
CBSE 12-science - Chemistry
Asked by harishshrivastava855 | 21 Jun, 2020, 21:26: PM
CBSE 12-science - Chemistry
Asked by manjurana04933 | 16 Apr, 2020, 11:00: AM
CBSE 12-science - Chemistry
Asked by Saransekar407 | 11 Mar, 2019, 18:47: PM
CBSE 12-science - Chemistry
Asked by yaag8432 | 05 Mar, 2019, 19:28: PM




