ICSE Class 7 Answered
take the mixture in a distillation flask and arrange the apparatus.
please mam explain in detail ...
Asked by jannatsavaliya8053.7sdatl | 19 Apr, 2020, 17:55: PM
PRINCIPLE: This method is used for the separation of components of a mixture containing two miscible liquids that boil without decomposition and have a sufficient difference in their boiling points.
TECHNIQUE: Take the mixture in a distillation flask. Fit the thermometer. Arrange the apparatus as shown in the given figure.
Heat the mixture slowly, keeping a close watch on the thermometer.
The liquid with a low boiling point will vaporise and condense in the condenser and can be collected from the condenser outlet.
The liquid with higher boiling point will be left behind in the distillation flask.
The vapours of the liquid are cooled to get pure liquid.
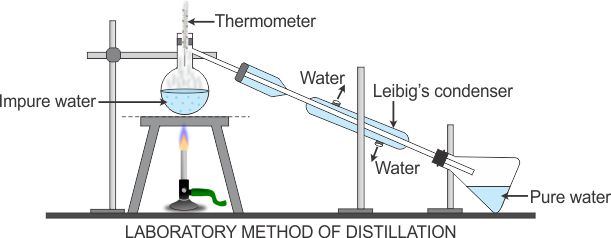
Distillation of water involves the boiling of water and the vapours form are condensed in condenser.
Condensation of water vapours gives pure water.
Answered by Ramandeep | 19 Apr, 2020, 19:48: PM
Concept Videos
ICSE 7 - Chemistry
Asked by krushnamundhe777 | 22 Oct, 2023, 13:11: PM
ICSE 7 - Chemistry
Asked by mitulraisa3784.7sdatl | 03 Jun, 2020, 14:22: PM
ICSE 7 - Chemistry
Asked by jayeshyashesh4969.7sdatl | 22 Apr, 2020, 10:51: AM
ICSE 7 - Chemistry
take the mixture in a distillation flask and arrange the apparatus.
please mam explain in detail ...
Asked by jannatsavaliya8053.7sdatl | 19 Apr, 2020, 17:55: PM
ICSE 7 - Chemistry
Asked by mishranilu907 | 21 Sep, 2018, 10:19: AM
ICSE 7 - Chemistry
Asked by sushma_chaudhari07 | 19 Aug, 2018, 11:20: AM
ICSE 7 - Chemistry
Asked by sushma_chaudhari07 | 19 Aug, 2018, 10:59: AM
ICSE 7 - Chemistry
Asked by sushma_chaudhari07 | 19 Aug, 2018, 10:55: AM
ICSE 7 - Chemistry
Asked by sushma_chaudhari07 | 19 Aug, 2018, 10:50: AM
ICSE 7 - Chemistry
Asked by sushma_chaudhari07 | 11 Aug, 2018, 12:17: PM






