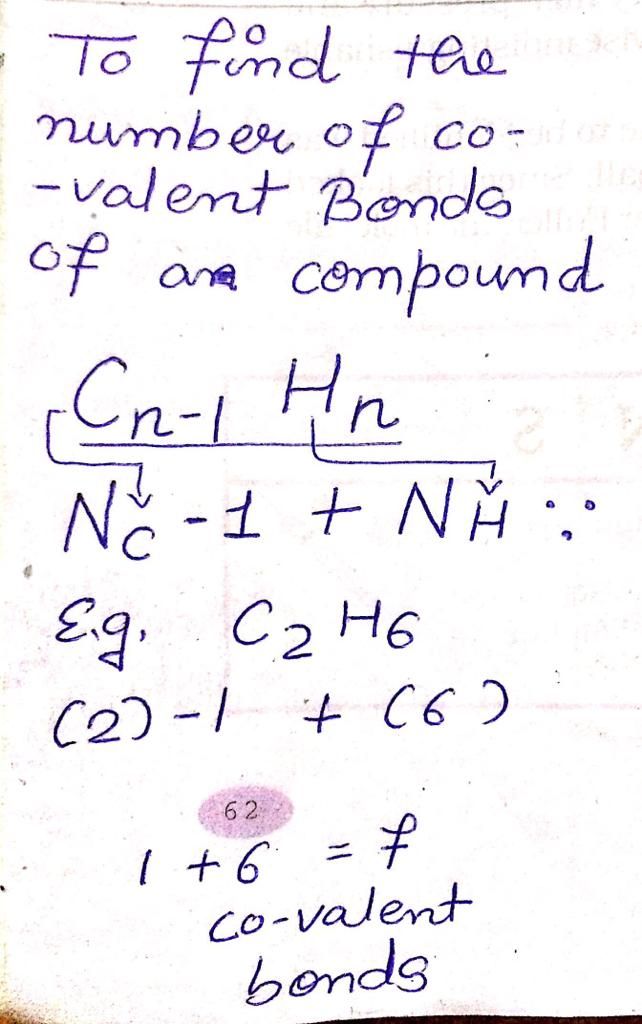
CBSE Class 10 Answered
Structure of diamond and graphite using vsepr theory
Asked by tialempongen177 | 09 Sep, 2020, 23:44: PM
Diamond
Diamond has a network structure consisting of a very large number of carbon atoms bonded to each other.
Each carbon atom is sp³ hybridised and is bonded to four other carbon atoms by single covalent bonds.
There is a three-dimensional network of strong covalent bonds in diamond which is very difficult to break.
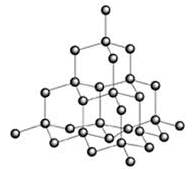
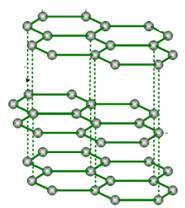
Graphite
In graphite, each carbon atom undergoes sp² hybridisation and is covalently bonded to three other carbon atoms by single bonds.
The fourth electron on each carbon atom forms bonds.
In this way, graphite consists of hexagonal rings in two dimensions.
The C–C covalent distance in rings is 141.5 pm, indicating strong bonding.
These arrays of rings form layers. The layers are separated by a distance of 340 pm.
Answered by Ramandeep | 10 Sep, 2020, 13:26: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by psaisruthi10012009 | 07 Jun, 2024, 11:09: AM
CBSE 10 - Chemistry
Asked by tialempongen177 | 09 Sep, 2020, 23:44: PM
CBSE 10 - Chemistry
Asked by seeni2005 | 05 Jul, 2020, 21:53: PM
CBSE 10 - Chemistry
Asked by subbukum | 04 Feb, 2020, 11:46: AM
CBSE 10 - Chemistry
Asked by navjotsinghdadwal | 01 Dec, 2019, 21:57: PM
CBSE 10 - Chemistry
Asked by 9886761796hmh | 17 Oct, 2019, 20:10: PM
CBSE 10 - Chemistry
Asked by ashishaman25082004 | 15 Sep, 2019, 22:01: PM
CBSE 10 - Chemistry
Asked by dr.sudhiguptajdmd74 | 23 Jul, 2019, 09:18: AM
CBSE 10 - Chemistry
Asked by labheshvaidya | 19 Apr, 2019, 15:38: PM
CBSE 10 - Chemistry
Asked by krishdabhoya2003 | 21 Feb, 2019, 17:52: PM