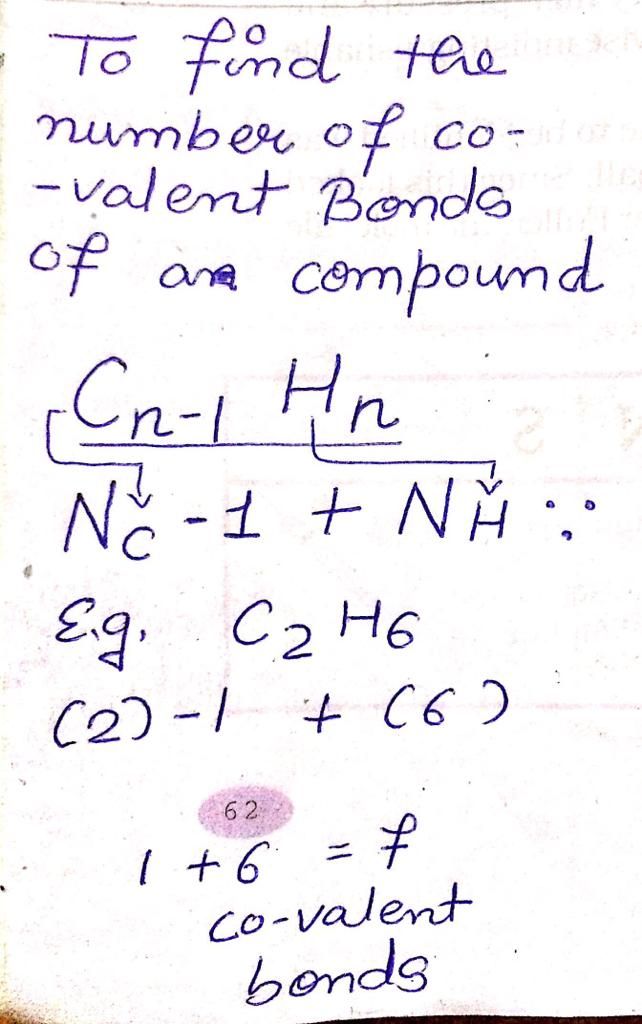
CBSE Class 10 Answered
Explain how the fullerenes can be made good conductor of electricity
Asked by navjotsinghdadwal | 01 Dec, 2019, 21:57: PM
Fullerene: It is an allotrope of carbon-containing clusters of 60 carbon atoms joined to form a spherical molecule. The C60 molecule has a shape like a soccer ball and is called Buckminsterfullerene. It contains 20 six-membered rings and 12 five-membered rings. All the carbon atoms are equal, and they undergo sp2 hybridisation. Each carbon atom forms three sigma bonds with three other carbon atoms. The remaining electron at each carbon is delocalised in molecular orbitals, which in turn give an aromatic character to the molecule. The pure form of fullerene acts as an insulator but can be converted into semiconductor or superconductor by doping under suitable conditions.
Answered by Ramandeep | 02 Dec, 2019, 11:16: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by psaisruthi10012009 | 07 Jun, 2024, 11:09: AM
CBSE 10 - Chemistry
Asked by tialempongen177 | 09 Sep, 2020, 23:44: PM
CBSE 10 - Chemistry
Asked by seeni2005 | 05 Jul, 2020, 21:53: PM
CBSE 10 - Chemistry
Asked by subbukum | 04 Feb, 2020, 11:46: AM
CBSE 10 - Chemistry
Asked by navjotsinghdadwal | 01 Dec, 2019, 21:57: PM
CBSE 10 - Chemistry
Asked by 9886761796hmh | 17 Oct, 2019, 20:10: PM
CBSE 10 - Chemistry
Asked by ashishaman25082004 | 15 Sep, 2019, 22:01: PM
CBSE 10 - Chemistry
Asked by dr.sudhiguptajdmd74 | 23 Jul, 2019, 09:18: AM
CBSE 10 - Chemistry
Asked by labheshvaidya | 19 Apr, 2019, 15:38: PM
CBSE 10 - Chemistry
Asked by krishdabhoya2003 | 21 Feb, 2019, 17:52: PM