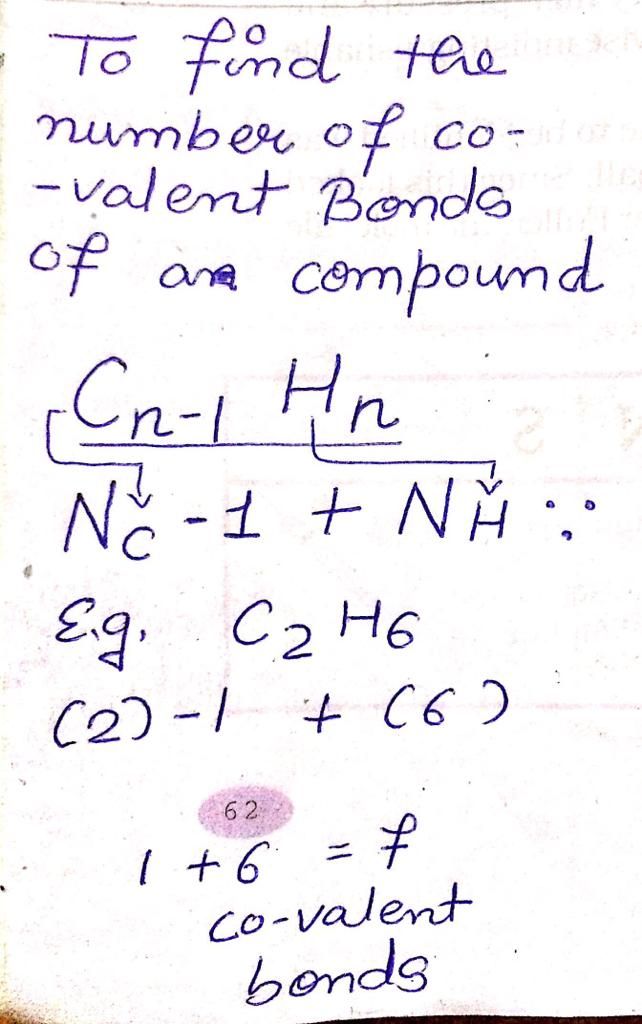
CBSE Class 10 Answered
A mixture of O2 and ethyne is used for welding purpose. Can you tell why a mixture of ethyne and air is not used?
Asked by subbukum | 04 Feb, 2020, 11:46: AM
Mixture of ethyne and air cannot be used due to hazard associated with ethyne's intrinsic instability; samples of concentrated or pure acetyle will explosively decompose in air. Acetylene can explode with extreme violence if the pressure of the gas exceeds about 200 kPa (≈39 psi) as a gas or when in liquid or solid form.
Answered by Ramandeep | 05 Feb, 2020, 12:47: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by psaisruthi10012009 | 07 Jun, 2024, 11:09: AM
CBSE 10 - Chemistry
Asked by tialempongen177 | 09 Sep, 2020, 23:44: PM
CBSE 10 - Chemistry
Asked by seeni2005 | 05 Jul, 2020, 21:53: PM
CBSE 10 - Chemistry
Asked by subbukum | 04 Feb, 2020, 11:46: AM
CBSE 10 - Chemistry
Asked by navjotsinghdadwal | 01 Dec, 2019, 21:57: PM
CBSE 10 - Chemistry
Asked by 9886761796hmh | 17 Oct, 2019, 20:10: PM
CBSE 10 - Chemistry
Asked by ashishaman25082004 | 15 Sep, 2019, 22:01: PM
CBSE 10 - Chemistry
Asked by dr.sudhiguptajdmd74 | 23 Jul, 2019, 09:18: AM
CBSE 10 - Chemistry
Asked by labheshvaidya | 19 Apr, 2019, 15:38: PM
CBSE 10 - Chemistry
Asked by krishdabhoya2003 | 21 Feb, 2019, 17:52: PM