CBSE Class 10 Answered
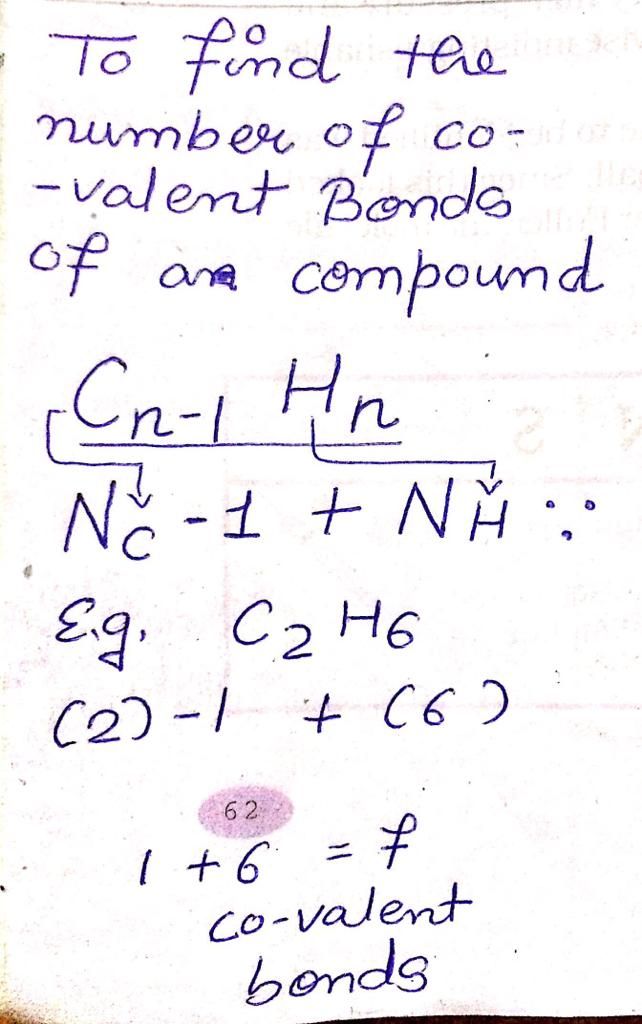
Show me the electron dot structure of CH2(Methylene).Also show the bonding.(2 or 3 marks)
Asked by dr.sudhiguptajdmd74 | 23 Jul, 2019, 09:18: AM
Carbenes: These are an electron deficient species with 6 electrons.

a. Triplet: They have 2 unpaired electrons in 2 different orbitals.
They may be either linear or bent, i.e. sp or sp2 hybridized, respectively.
b. Singlet: They have two paired electrons in a single orbital.
Singlet carbenes are spin-paired and adopt a sp2 hybrid structure

Answered by Ramandeep | 23 Jul, 2019, 10:54: AM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by psaisruthi10012009 | 07 Jun, 2024, 11:09: AM
CBSE 10 - Chemistry
Asked by tialempongen177 | 09 Sep, 2020, 23:44: PM
CBSE 10 - Chemistry
Asked by seeni2005 | 05 Jul, 2020, 21:53: PM
CBSE 10 - Chemistry
Asked by subbukum | 04 Feb, 2020, 11:46: AM
CBSE 10 - Chemistry
Asked by navjotsinghdadwal | 01 Dec, 2019, 21:57: PM
CBSE 10 - Chemistry
Asked by 9886761796hmh | 17 Oct, 2019, 20:10: PM
CBSE 10 - Chemistry
Asked by ashishaman25082004 | 15 Sep, 2019, 22:01: PM
CBSE 10 - Chemistry
Asked by dr.sudhiguptajdmd74 | 23 Jul, 2019, 09:18: AM
CBSE 10 - Chemistry
Asked by labheshvaidya | 19 Apr, 2019, 15:38: PM
CBSE 10 - Chemistry
Asked by krishdabhoya2003 | 21 Feb, 2019, 17:52: PM