CBSE Class 10 Answered
lewis dot structure and explanation of H2SO4 and CO
Asked by ashishaman25082004 | 15 Sep, 2019, 22:01: PM
Follow these simple steps to draw dot structures:
- Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons.
- If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, and subtract an electrons for every positive (+) charge.
- Consider bonding between atoms by sharing electrons, some may come from one atom.
- If possible, apply the octet rule to your structure. Some structures don't obey the octet rule, but explain why.
- Assign formal charges to atoms in the structure.
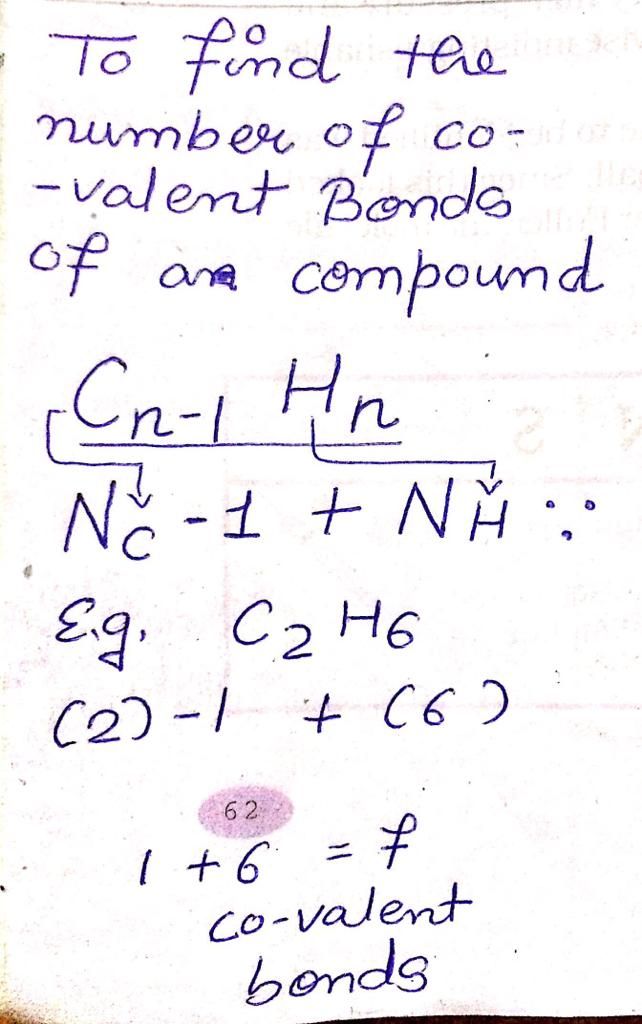
Let us consider the electron dot structure of sulphuric acid,
The chemical formula of Sulpuric acid is H2SO4:
Sulphur atom (2, 8, 6) is the central atom have six valence electrons, as it has vacant d-orbital sulphur can show variable valencies.
And for CO:
Answered by Ramandeep | 16 Sep, 2019, 15:48: PM
Application Videos
Concept Videos
CBSE 10 - Chemistry
Asked by psaisruthi10012009 | 07 Jun, 2024, 11:09: AM
CBSE 10 - Chemistry
Asked by tialempongen177 | 09 Sep, 2020, 23:44: PM
CBSE 10 - Chemistry
Asked by seeni2005 | 05 Jul, 2020, 21:53: PM
CBSE 10 - Chemistry
Asked by subbukum | 04 Feb, 2020, 11:46: AM
CBSE 10 - Chemistry
Asked by navjotsinghdadwal | 01 Dec, 2019, 21:57: PM
CBSE 10 - Chemistry
Asked by 9886761796hmh | 17 Oct, 2019, 20:10: PM
CBSE 10 - Chemistry
Asked by ashishaman25082004 | 15 Sep, 2019, 22:01: PM
CBSE 10 - Chemistry
Asked by dr.sudhiguptajdmd74 | 23 Jul, 2019, 09:18: AM
CBSE 10 - Chemistry
Asked by labheshvaidya | 19 Apr, 2019, 15:38: PM
CBSE 10 - Chemistry
Asked by krishdabhoya2003 | 21 Feb, 2019, 17:52: PM