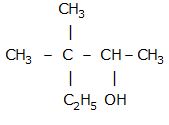CBSE Class 12-science Answered
plz telll why propanol has higher boiling point than that of the hydrocarbon butane?
Asked by manishasharma1912 | 17 Jun, 2014, 07:42: AM
Propanol can form intermolecular H-bonding due to which its boling point is higher than butane which is non-polar.
Answered by Vaibhav Chavan | 17 Jun, 2014, 05:33: PM
Concept Videos
CBSE 12-science - Chemistry
Asked by akhilmaigur987 | 20 May, 2020, 02:55: PM
CBSE 12-science - Chemistry
Asked by aayushkumargupta3 | 10 Apr, 2020, 10:56: PM
CBSE 12-science - Chemistry
Asked by chauhanparth974 | 08 Mar, 2020, 09:34: AM
CBSE 12-science - Chemistry
Asked by ajaysankhala051 | 03 Mar, 2020, 11:33: PM
CBSE 12-science - Chemistry
Asked by pfffxy571 | 08 Jun, 2018, 10:27: AM
CBSE 12-science - Chemistry
Asked by Girijeshpandey.rjil | 23 Apr, 2018, 07:16: PM
CBSE 12-science - Chemistry
Asked by hbohra67 | 04 Apr, 2018, 03:53: AM
CBSE 12-science - Chemistry
Asked by Balbir | 03 Apr, 2018, 08:26: PM
CBSE 12-science - Chemistry
Asked by raul.chintakindi14 | 14 Mar, 2018, 11:58: AM
CBSE 12-science - Chemistry
Asked by Topperlearning User | 24 Mar, 2014, 09:00: AM