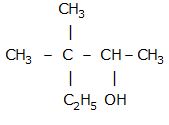CBSE Class 12-science Answered
How does decrease in surface area of branched alcohols decrease vander walls forces?
Asked by Girijeshpandey.rjil | 23 Apr, 2018, 19:16: PM
Let us understand with an example,
2,2 dimethyl propane is a compact, almost spherical molecule whereas, pentane is long and floppier so will experience more forces between molecules.

In above structures, you can see that n-pentane have larger surface area than 2,2 dimethylpropane, which means the Van der Waal's forces of attraction are more in case of n-pentane than 2,2 dimethyl propane.
Answered by Ramandeep | 24 Apr, 2018, 10:38: AM
Concept Videos
CBSE 12-science - Chemistry
Asked by guneetk44 | 28 Apr, 2024, 10:39: AM
CBSE 12-science - Chemistry
Asked by akhilmaigur987 | 20 May, 2020, 14:55: PM
CBSE 12-science - Chemistry
Asked by aayushkumargupta3 | 10 Apr, 2020, 22:56: PM
CBSE 12-science - Chemistry
Asked by chauhanparth974 | 08 Mar, 2020, 09:34: AM
CBSE 12-science - Chemistry
Asked by ajaysankhala051 | 03 Mar, 2020, 23:33: PM
CBSE 12-science - Chemistry
Asked by pfffxy571 | 08 Jun, 2018, 10:27: AM
CBSE 12-science - Chemistry
Asked by Girijeshpandey.rjil | 23 Apr, 2018, 19:16: PM
CBSE 12-science - Chemistry
Asked by hbohra67 | 04 Apr, 2018, 03:53: AM
CBSE 12-science - Chemistry
Asked by Balbir | 03 Apr, 2018, 20:26: PM
CBSE 12-science - Chemistry
Asked by raul.chintakindi14 | 14 Mar, 2018, 11:58: AM