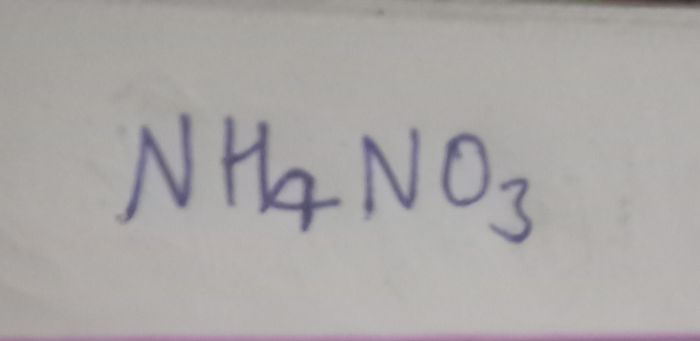CBSE Class 12-science Answered
please give structures of hybrids of SO2 and explain the formation of each briefly
Asked by kanwal sandhu | 21 Jan, 2012, 08:34: PM
We are not getting what is the question. what are hybrids in chemistry?
If you want to know about hybridisation it is as follows:
Sulfur has 6 valence electrons and so does oxygen, so in SO2, we're dealing with 18valence electrons total. First, draw single bonds between the S and each O, then fill out an octet for each O. This uses 16 electrons. The other two should be placed as a lone pair on the S. Finally, move a lone pair on one of the O's so that it forms a second bond with the S. This completes an octet for all atoms. Now count electrons groups around the S: there's a double bond, a single bond, and a lone pair.... that makes 3. So the S in SO2 is sp2 hybridized.
Answered by | 22 Jan, 2012, 11:28: AM
Application Videos
Concept Videos
CBSE 12-science - Chemistry
Asked by shwetayaligar205 | 21 Dec, 2022, 07:45: PM
CBSE 12-science - Chemistry
Asked by kushwaharitik9129 | 14 Jul, 2022, 01:06: PM
CBSE 12-science - Chemistry
Asked by gurugubellisaivishal2705 | 09 Jul, 2022, 12:16: PM
CBSE 12-science - Chemistry
Asked by nonuhasan2 | 20 Nov, 2021, 12:05: PM
CBSE 12-science - Chemistry
Asked by skumkum976 | 08 May, 2021, 03:49: PM
CBSE 12-science - Chemistry
Asked by cute44464 | 01 Mar, 2021, 01:17: PM
CBSE 12-science - Chemistry
Asked by manivannanbalakrishnan52 | 09 Dec, 2020, 10:06: PM
CBSE 12-science - Chemistry
Asked by onkaronkar618 | 12 Oct, 2020, 11:38: PM
CBSE 12-science - Chemistry
Asked by contactus.topperlearning | 13 Sep, 2020, 01:21: PM
CBSE 12-science - Chemistry
Asked by nishaohlyan835 | 30 Jun, 2020, 04:18: PM