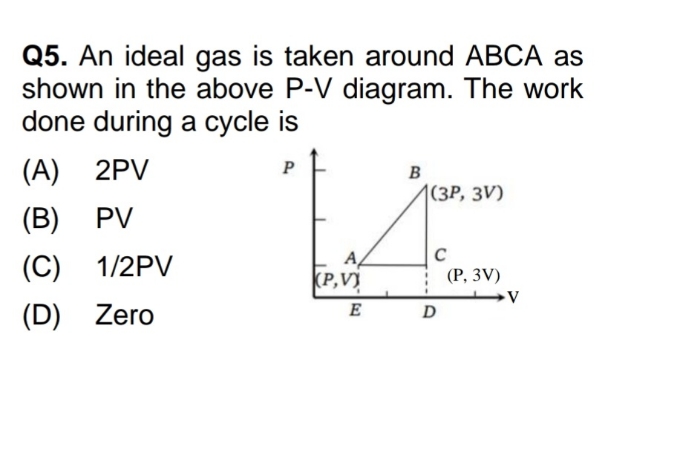
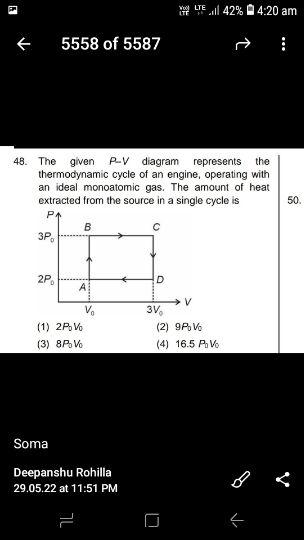
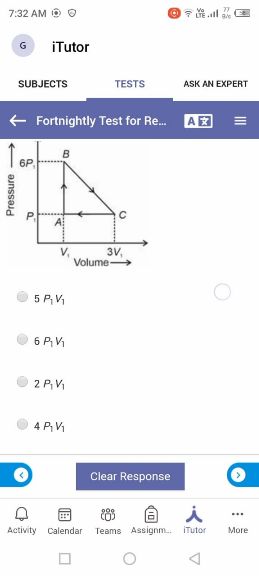
NEET Class neet Answered
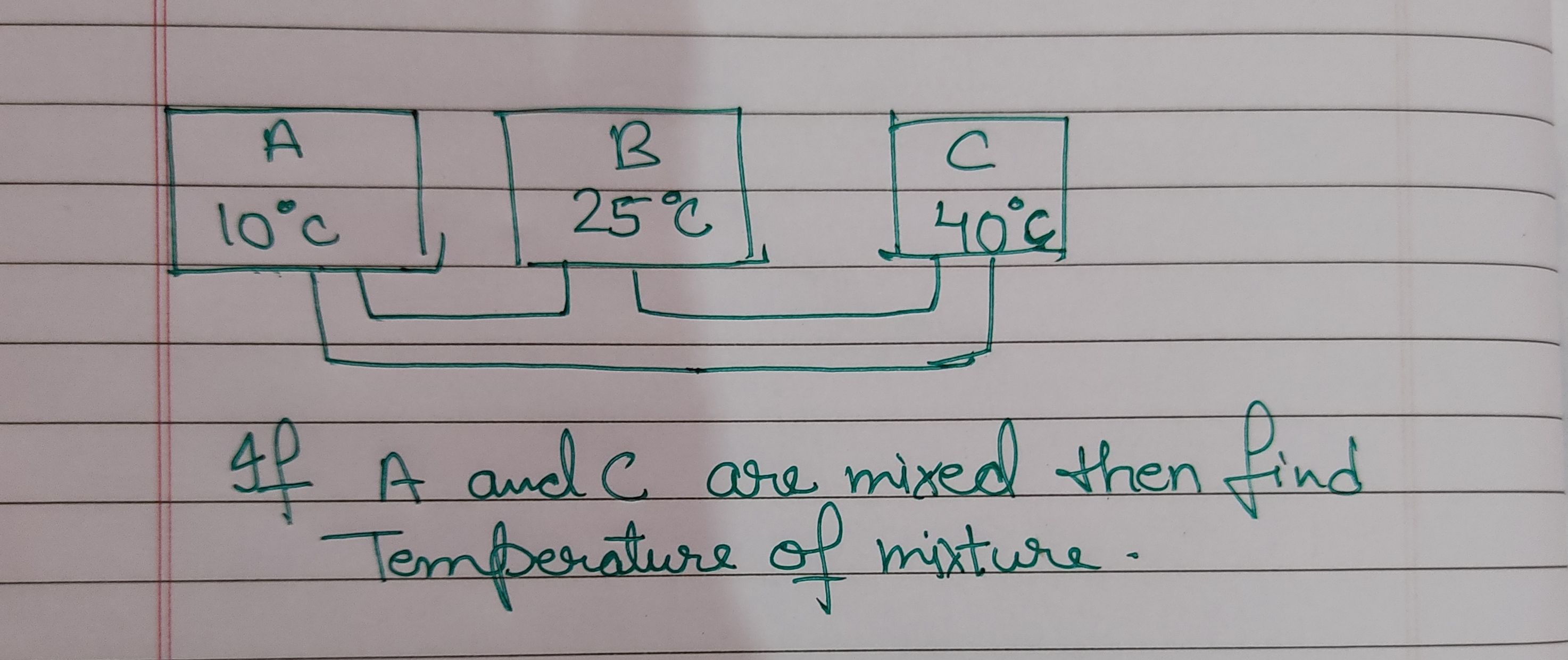
Please explain how to solve this
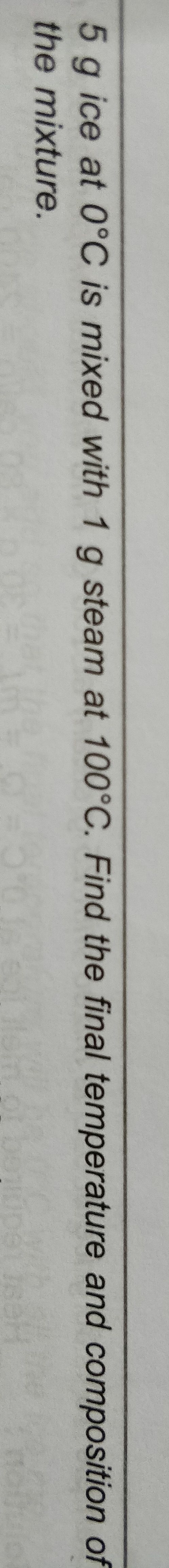
Asked by 22bakugoku | 21 Apr, 2019, 17:23: PM
Latent heat of ice = m×l = 5×80 = 400cal
Latent heat of steam = 1×540 = 540cal
Here the heat release by steam is more so it will melt down ice completely but still 540-400 = 140 cal energy is left
This will increase the temperature of ice from 0 celcius
So let us assume final temperature to be T
So 5×1×T = 140+ 1×1×T
4T = 140
So T = 140/4 = 35 celcius
Amount of water = 5+1 = 6gm
Answered by Ankit K | 21 Apr, 2019, 22:44: PM
NEET neet - Physics
Asked by yadavaradhana9335 | 19 Feb, 2024, 16:53: PM
NEET neet - Physics
Asked by sujitjana971 | 18 Dec, 2022, 17:23: PM
NEET neet - Physics
Asked by takshitashu46 | 09 Feb, 2022, 18:01: PM
NEET neet - Physics
Asked by jhajuhi19 | 18 Aug, 2021, 03:21: AM
NEET neet - Physics
Asked by 22bakugoku | 21 Apr, 2019, 17:23: PM
NEET neet - Physics
Asked by 22bakugoku | 21 Apr, 2019, 17:23: PM