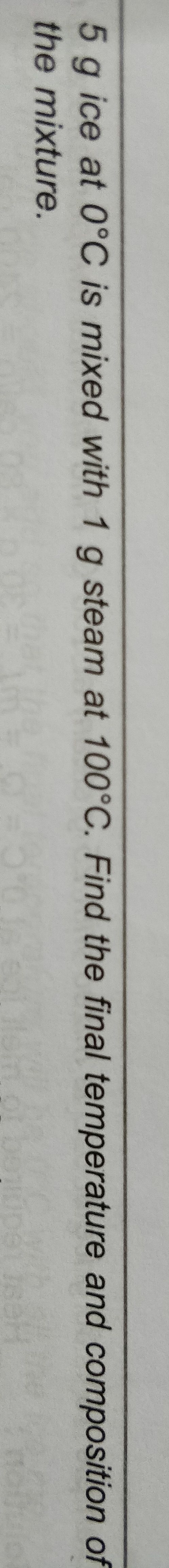NEET Class neet Answered
how we find work done

Asked by sujitjana971 | 18 Dec, 2022, 17:23: PM
Given that,
ΔQ=80 J
For mono atomic gas,
And for isobaric process (Constant pressure), ΔQ = nCpΔT and ΔW = nRΔT
Thus,
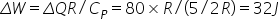
Now, according to first law of thermodynamics
ΔE =ΔQ – ΔW = 80 – 32 = 48 J
Answered by Jayesh Sah | 18 Dec, 2022, 20:11: PM
NEET neet - Physics
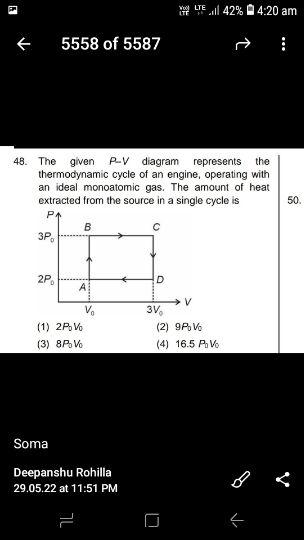
Asked by yadavaradhana9335 | 19 Feb, 2024, 16:53: PM
NEET neet - Physics
Asked by sujitjana971 | 18 Dec, 2022, 17:23: PM
NEET neet - Physics
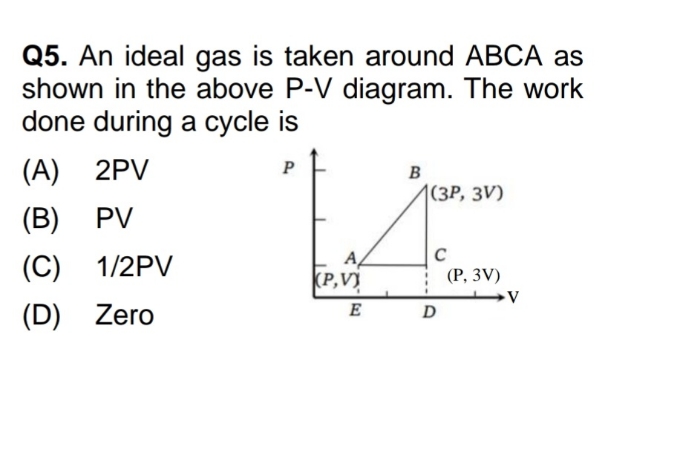
Asked by takshitashu46 | 09 Feb, 2022, 18:01: PM
NEET neet - Physics
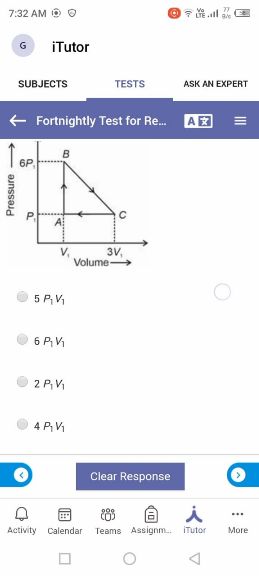
Asked by jhajuhi19 | 18 Aug, 2021, 03:21: AM
NEET neet - Physics
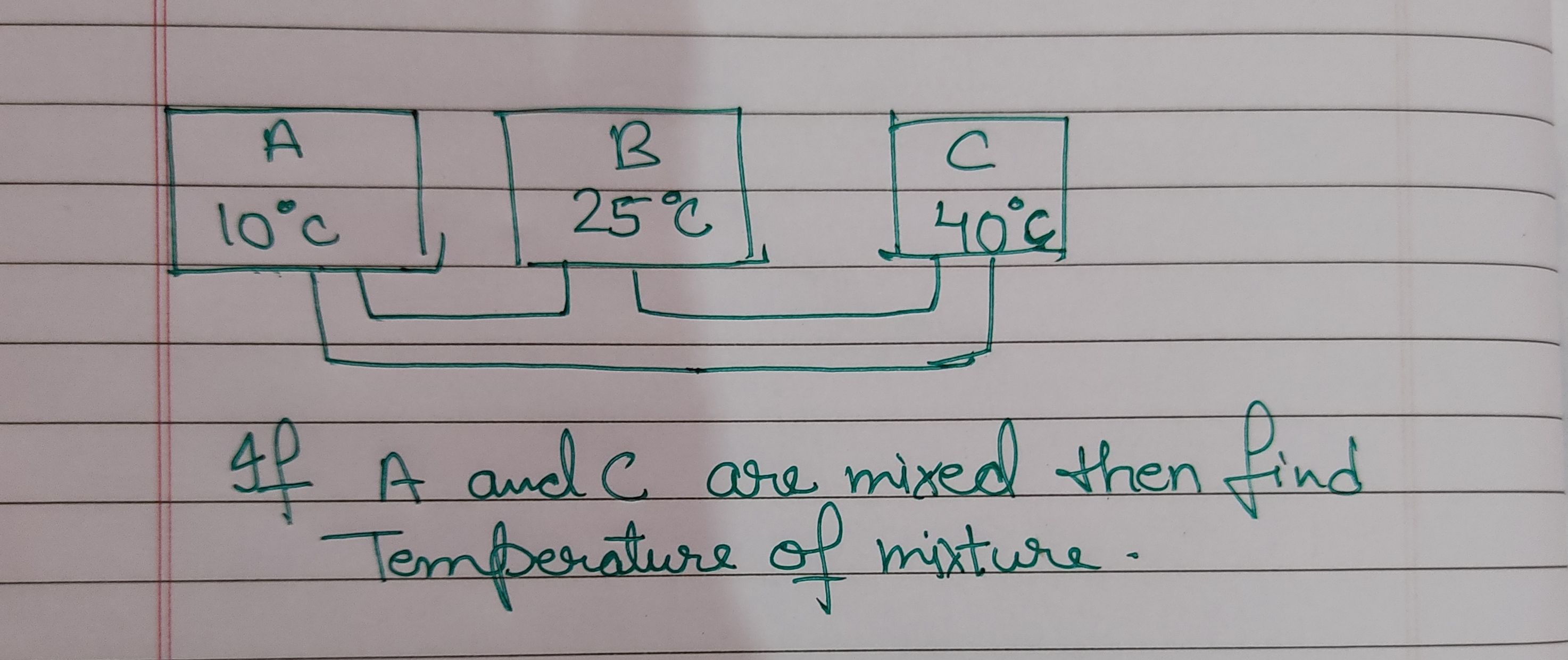
Asked by 22bakugoku | 21 Apr, 2019, 17:23: PM
NEET neet - Physics
Asked by 22bakugoku | 21 Apr, 2019, 17:23: PM