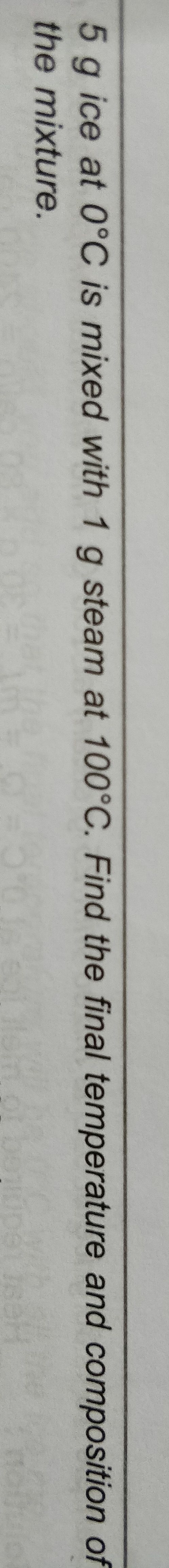NEET Class neet Answered
Please explain how to do this

Asked by 22bakugoku | 21 Apr, 2019, 17:23: PM
Latent heat of ice =m×l 100×80=8000 cal
Latent heat of steam =m×l =10×540 = 5400cal
Latent Heat of ice is greater than steam
Specific heat of steam = 10× 1×100 = 1000 cal
Net heat released by steam = 5400+1000= 6400 cal
Latent heat of ice is greater than steam.
So only 6400 cal equivalent of ice gonna melt
So mass of melted ice (water will still be at 0 celcius) = 6400/80 = 80gm
So 80gm of ice will melt into water
Net water = 80 +10(steam) = 90gm
Answered by Ankit K | 21 Apr, 2019, 22:36: PM
NEET neet - Physics
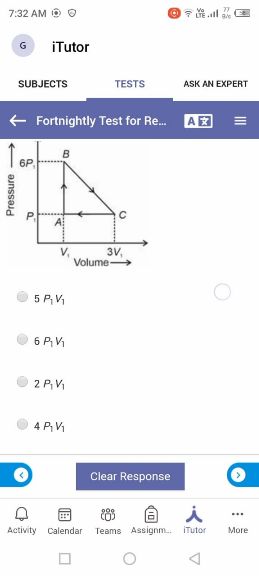
Asked by yadavaradhana9335 | 19 Feb, 2024, 16:53: PM
NEET neet - Physics
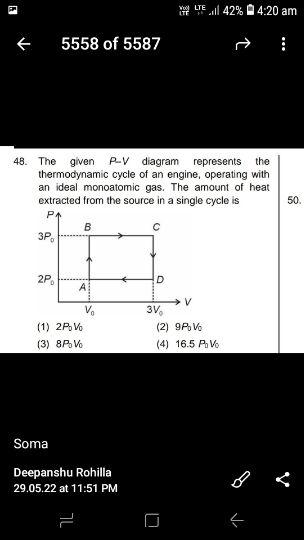
Asked by sujitjana971 | 18 Dec, 2022, 17:23: PM
NEET neet - Physics
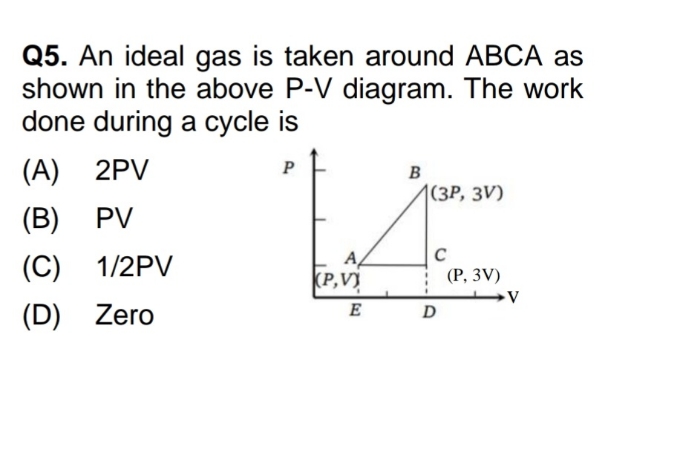
Asked by takshitashu46 | 09 Feb, 2022, 18:01: PM
NEET neet - Physics
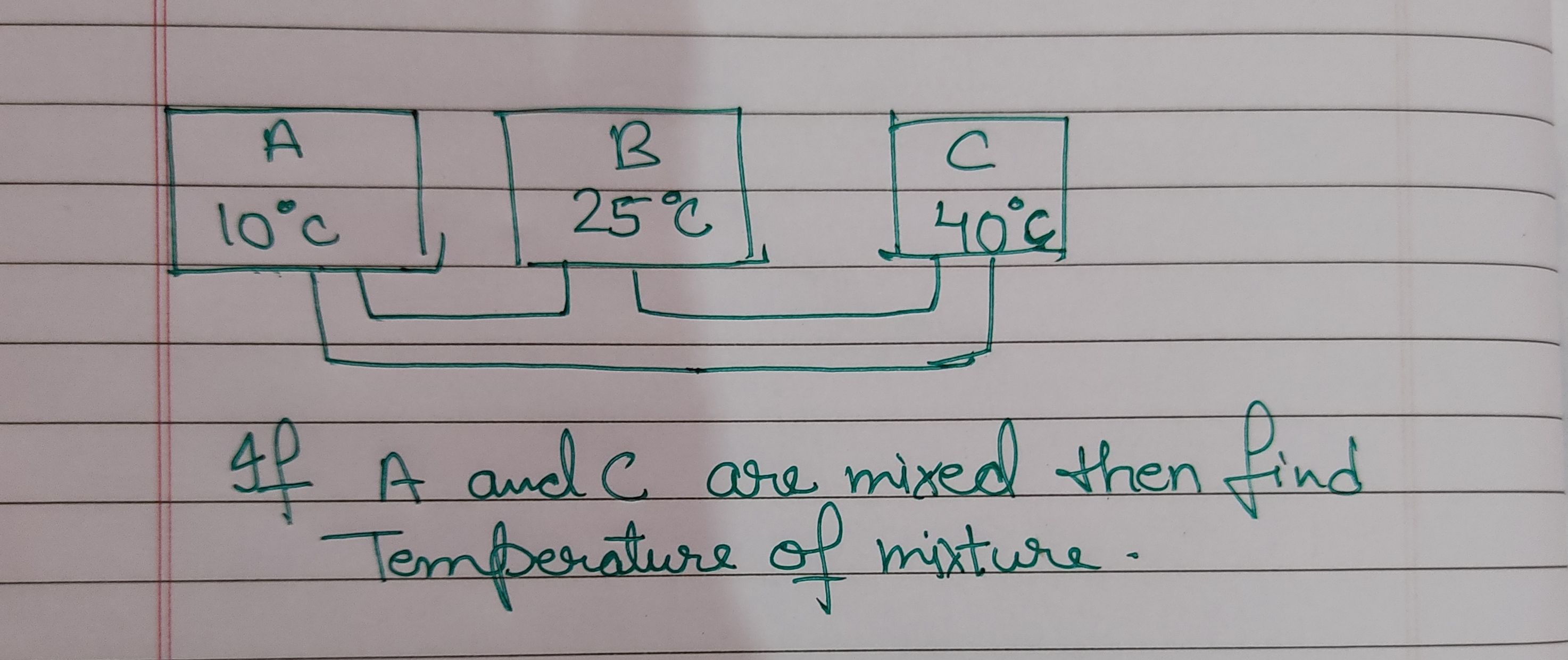
Asked by jhajuhi19 | 18 Aug, 2021, 03:21: AM
NEET neet - Physics
Asked by 22bakugoku | 21 Apr, 2019, 17:23: PM
NEET neet - Physics
Asked by 22bakugoku | 21 Apr, 2019, 17:23: PM