NEET Class neet Answered
Heat required to convert one gram of ice at 0 Celsius into steam at 100 Celsius
Asked by takshitashu46 | 09 Feb, 2022, 18:01: PM
Heat Q1 required to convert 1 g ice at 0o C to water at 0o C is given as
Q1 = m × Lf = 10-3 × 336 × 103 = 336 J ........................... (1)
where m is mass of ice , Lf is latent heat of fusion of ice .
Heat Q2 required to convert 1 g water at 0o C to water at 100o C is given as
Q2 = m × Cp × 100 = 10-3 × 4186 × 100 ≈ 419 J ...................... (2)
Heat Q3 required to convert 1 g water at 100o C to steam at 100o C is given as
Q3 = m × Lv = 10-3 × 2258 × 103 = 2258 J
Hence, total heat = Q1 + Q2 + Q3 = ( 336 + 419 + 2258 ) J = 3013 J
Answered by Thiyagarajan K | 10 Feb, 2022, 00:12: AM
NEET neet - Physics
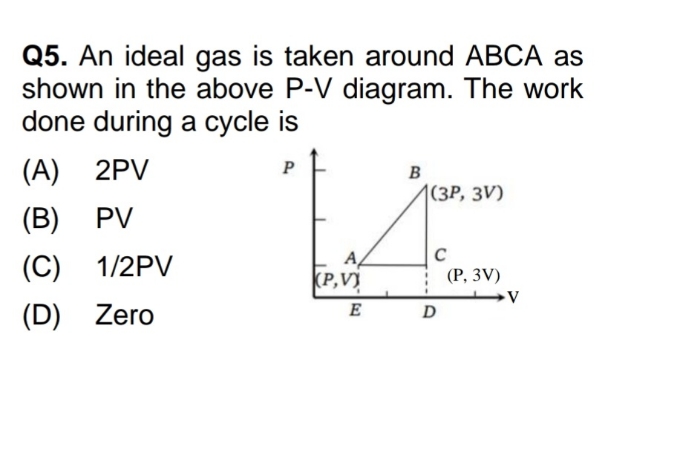
Asked by yadavaradhana9335 | 19 Feb, 2024, 16:53: PM
NEET neet - Physics
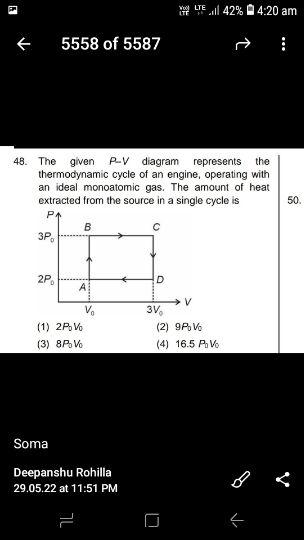
Asked by sujitjana971 | 18 Dec, 2022, 17:23: PM
NEET neet - Physics
Asked by takshitashu46 | 09 Feb, 2022, 18:01: PM
NEET neet - Physics
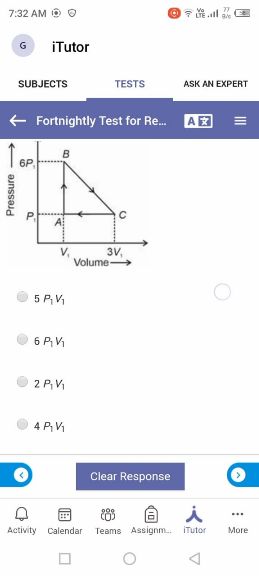
Asked by jhajuhi19 | 18 Aug, 2021, 03:21: AM
NEET neet - Physics
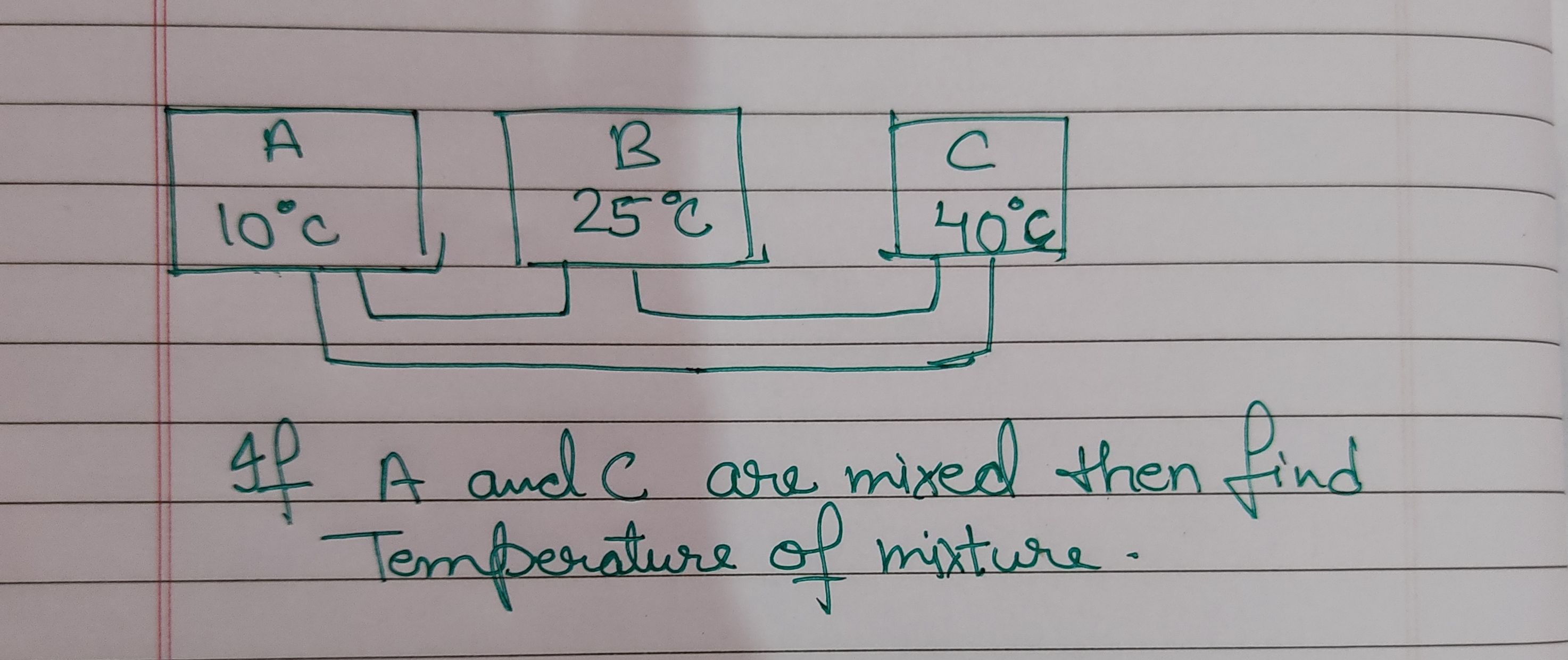
Asked by 22bakugoku | 21 Apr, 2019, 17:23: PM
NEET neet - Physics
Asked by 22bakugoku | 21 Apr, 2019, 17:23: PM