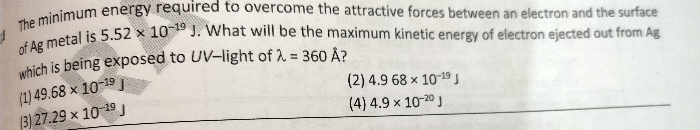CBSE Class 11-science Answered
like Cr and Cu ,why Si dont have the electronic configuration of 3s1and 3p3
Asked by stuti jain | 28 Jul, 2011, 12:00: AM
The reason behind this is the energy gap between the orbitals.
hope this figure will help in understanding this.
In case of Cu, the electrons are filled as 3d10 4s1. This is based on Hund's rule.
Electrons fill low energy orbitals (closer to the nucleus) before they fill higher energy ones. Where there is a choice between orbitals of equal energy, they fill the orbitals singly as far as possible.
This filling of orbitals singly where possible is known as Hund's rule. It only applies where the orbitals have exactly the same energies (as with p orbitals, for example), and helps to minimise the repulsions between electrons and so makes the atom more stable.
Answered by | 01 Aug, 2011, 02:30: PM
Application Videos
Concept Videos
CBSE 11-science - Chemistry
Asked by saranyachakraborty2007 | 25 Apr, 2024, 05:23: AM
CBSE 11-science - Chemistry
Asked by ammu32811 | 20 Feb, 2024, 08:58: AM
CBSE 11-science - Chemistry
Asked by ee7511641 | 13 Jan, 2024, 03:37: PM
CBSE 11-science - Chemistry
Asked by kv3582976 | 11 Oct, 2023, 06:57: AM
CBSE 11-science - Chemistry
Asked by o230397 | 23 Sep, 2023, 02:48: PM
CBSE 11-science - Chemistry
Asked by shrreya27harshitha | 17 Jul, 2022, 04:15: PM
CBSE 11-science - Chemistry
Asked by habibakhatoon112 | 15 Jul, 2022, 09:14: PM
CBSE 11-science - Chemistry
Asked by deba.biswas561 | 14 Jun, 2022, 08:07: AM
CBSE 11-science - Chemistry
Asked by advssdrall | 12 Jan, 2022, 05:12: AM
CBSE 11-science - Chemistry
Asked by ks1221516 | 14 Nov, 2021, 06:46: PM