CBSE Class 11-science Answered
sir what is magnetic quantum No.
Asked by ks1221516 | 14 Nov, 2021, 18:46: PM
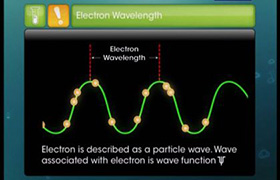
The magnetic quantum number is the third on the list between spin and azimuthal quantum number. It splits the sub-shells (such as s,p,d,f) into individual orbitals and places the electron in one of them. For example, s sub-shell has only one orbital, the p sub-shell has 3 and d has 5, and so on.
Answered by Sheetal Kolte | 15 Nov, 2021, 12:29: PM
Concept Videos
CBSE 11-science - Chemistry
Asked by o230397 | 23 Sep, 2023, 14:48: PM
CBSE 11-science - Chemistry
Asked by ks1221516 | 14 Nov, 2021, 18:46: PM
CBSE 11-science - Chemistry
Does shielding effect occur between atomic orbitals of the same quantum number? Explain with reasons
Asked by fishtailfever | 08 Sep, 2019, 23:00: PM
CBSE 11-science - Chemistry
Asked by patra04011965 | 09 Aug, 2019, 17:19: PM
CBSE 11-science - Chemistry
Asked by bhrjitu | 21 Jul, 2019, 08:08: AM
CBSE 11-science - Chemistry
Asked by pb_ckt | 06 Jun, 2019, 09:15: AM
CBSE 11-science - Chemistry
Asked by shahrithik07 | 18 Oct, 2018, 17:26: PM
CBSE 11-science - Chemistry
Asked by chinjalsoni911 | 16 Oct, 2018, 18:14: PM
CBSE 11-science - Chemistry
Asked by arunavamitra50 | 18 Jun, 2018, 18:39: PM
CBSE 11-science - Chemistry
Asked by badalsharma9929 | 13 Jun, 2018, 08:43: AM